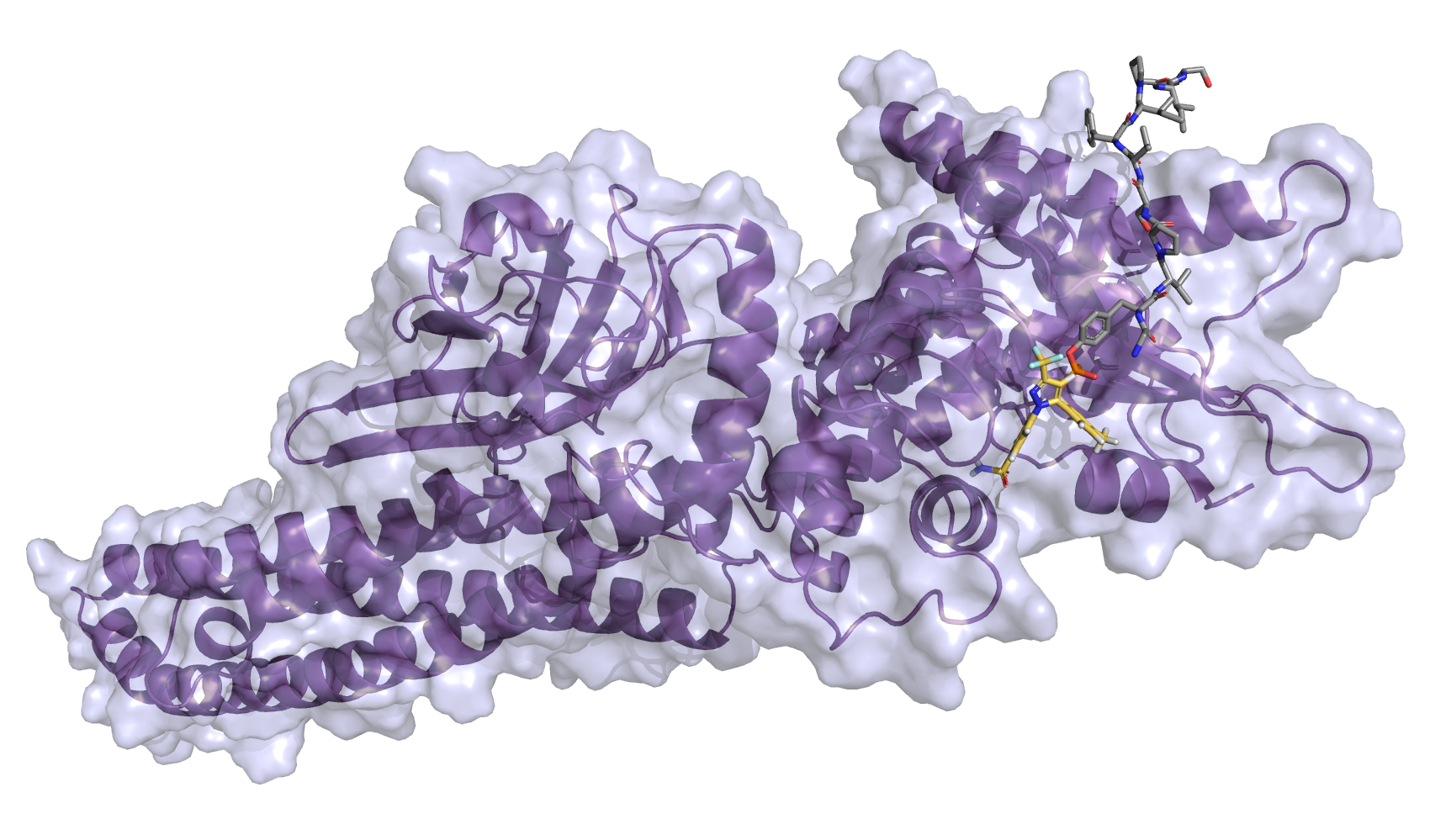
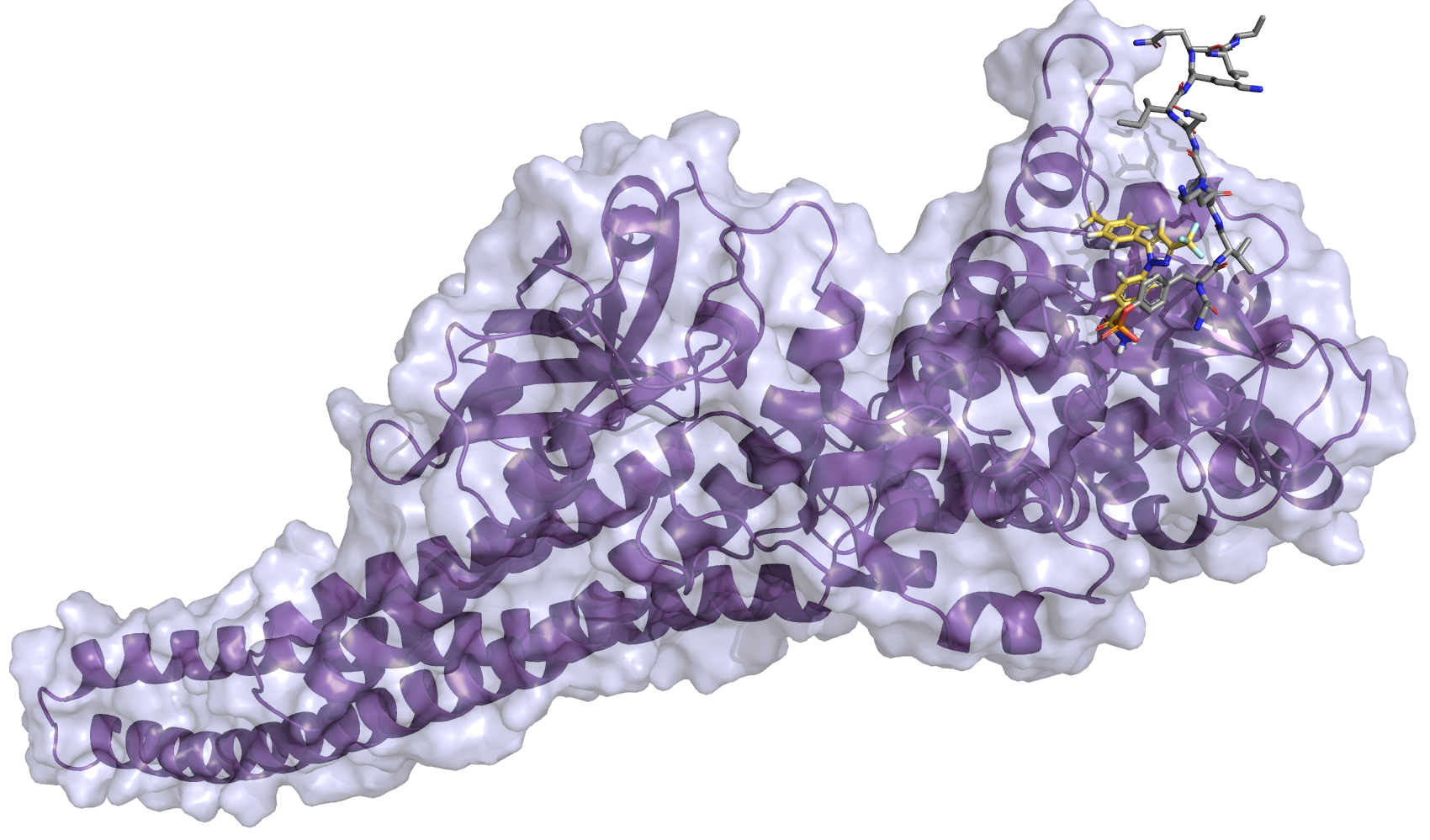
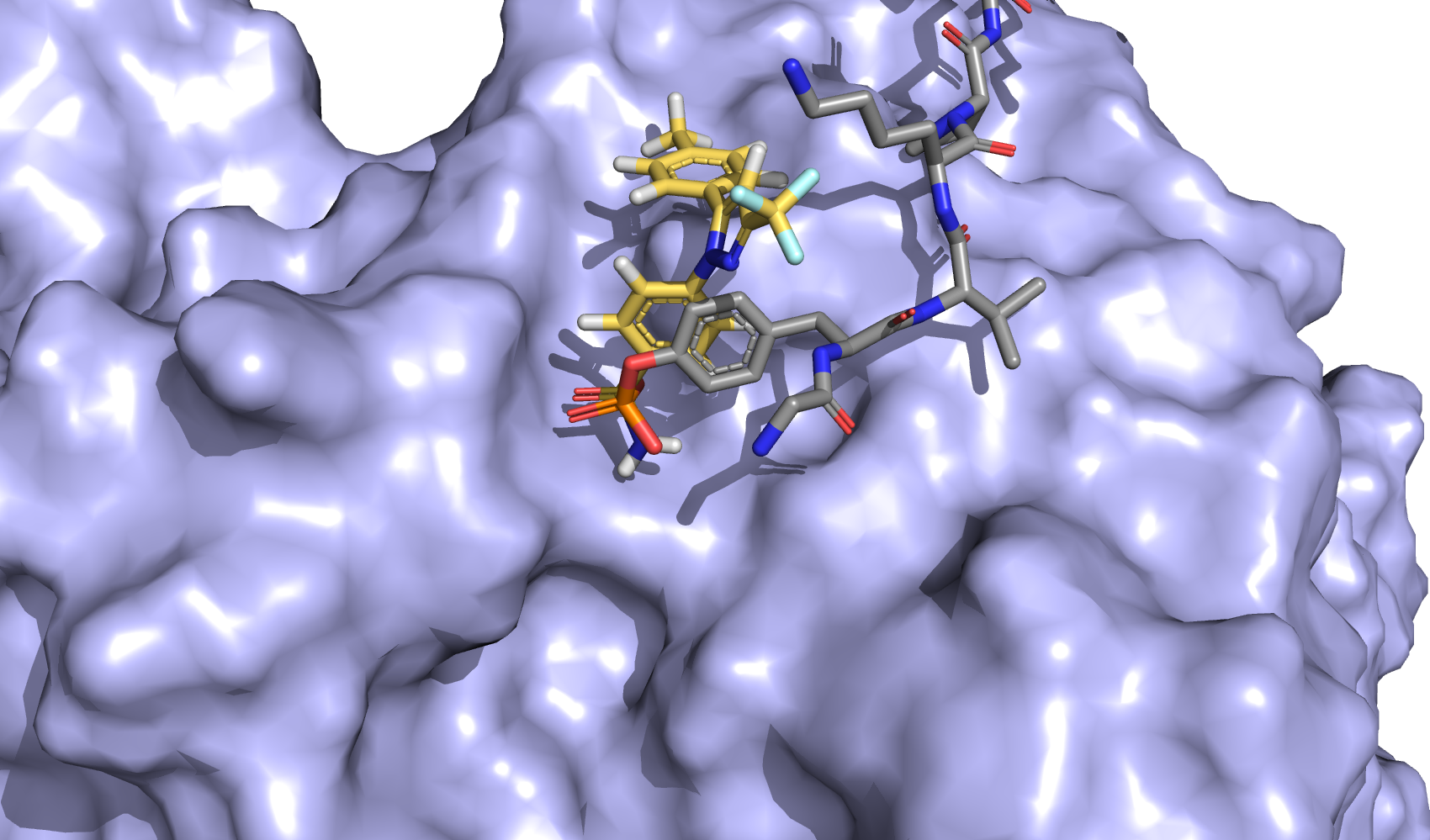
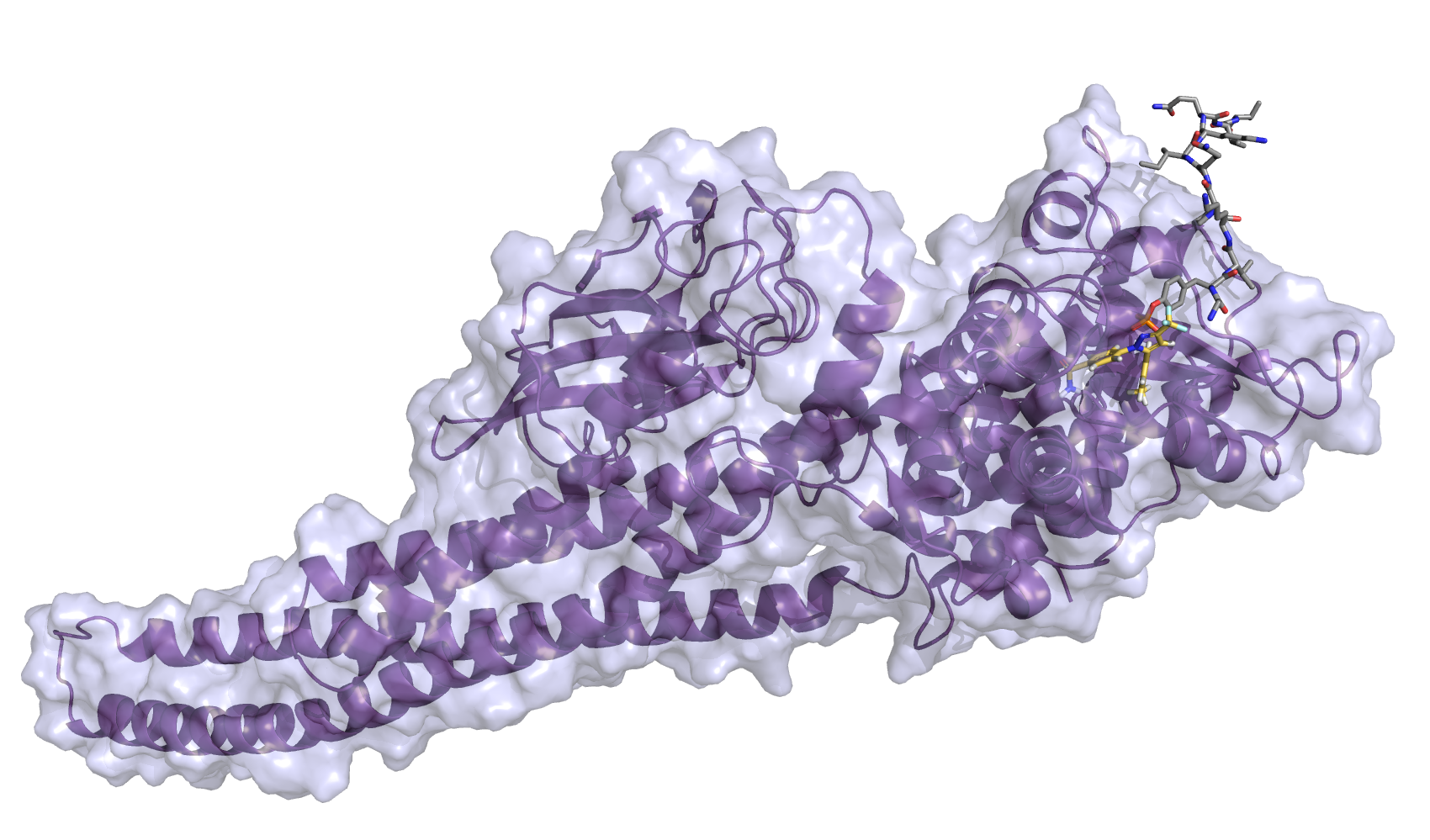
| Intravitreal Celecoxib for Chronic Uveitis |
Terminated |
Inflammation |
NCT02131012 |
| Celecoxib Window of Opportunity Trial to Assess Tumor and Stroma Responses |
Withdrawn |
Breast Carcinoma |
NCT03185871 |
| Neoadjuvant Celecoxib in Newly Diagnosed Patients With Endometrial Carcinoma |
Recruiting |
Endometrium Cancer |
NCT03896113 |
| European Celecoxib Trial in Primary Breast Cancer |
Completed |
Breast Cancer |
NCT02429427 |
| Randomized Controlled Phase II Trial of Pre-operative Celecoxib Treatment in Breast Cancer |
Completed |
Breast Neoplasms|Breast Cancer |
NCT01695226 |
| Lipidomics Screening of Celecoxib in ex Vivo Human Whole Blood Assay - Part B |
Completed |
Healthy |
NCT02413203 |
| The Study to Evaluate Efficacy and Safety of Celecoxib Capsule in Hand Osteoarthritis Patients |
Completed |
Osteoarthritis Hand |
NCT03067194 |
| The Impact of Genotype on Plasma and Cerebral Spinal Fluid Pharmacokinetics of Celecoxib in Children |
Not yet recruiting |
Pharmacokinetics of Celecoxib in Children |
NCT01344200 |
| Effect of Low-Dose Celecoxib on SMN2 in Patients With Spinal Muscular Atrophy |
Recruiting |
Spinal Muscular Atrophy (SMA) |
NCT02876094 |
| Evaluation of Perioperative Celecoxib for Hip Arthroscopy |
Completed |
Hip Labral Tears |
NCT02779166 |
| Adjunctive Use of Celecoxib in the Treatment of Bipolar Postpartum Depression |
Recruiting |
Bipolar Disorder|Postpartum Depression |
NCT02726659 |
| An Open Label Phase II Study Combining Nivolumab and Celecoxib in Patients With Advanced " Cold " Solid Tumors |
Not yet recruiting |
Metastatic Cancer |
NCT03864575 |
| Phase I Study of Episcleral Celecoxib for Treatment of Macular Edema and Inflammatory Disorders of the Posterior Pole |
Recruiting |
Macula Edema|Radiation Retinopathy|Branch Retinal Vein Occlusion|Epiretinal Membrane|Central Serous Retinopathy With Pit of Optic Disc|Commotio Retinae|Vitritis |
NCT04120636 |
| Celecoxib in Colposcopic Directed Biopsy |
Recruiting |
Colposcopy |
NCT03464552 |
| Concurrent Radiotherapy and/or Cisplatin With or Without Celecoxib in Patients With Primary Oral Squamous Cell Carcinoma |
Unknown status |
Oral Squamous Cell Carcinoma |
NCT02739204 |
| Effect of AKB-6548 on the Pharmacokinetics of Celecoxib |
Completed |
Healthy Volunteers |
NCT02502500 |
| Celecoxib for Thyroid Eye Disease |
Recruiting |
Thyroid Eye Disease |
NCT02845336 |
| Celecoxib Inhibition of Aromatase Expression and Inflammation in Adipose Tissue of Obese Postmenopausal Women |
Completed |
Obesity |
NCT01901679 |
| Effect of Celecoxib on Postoperative Analgesia and Disease Severity in AERD Patients With CRS |
Recruiting |
Chronic Rhinosinusitis With Nasal Polyps|Aspirin-exacerbated Respiratory Disease |
NCT04147013 |
| Celecoxib vs. Acetaminophen/Codeine/Caffeine for Post-operative Analgesia in Rhinoplasty. |
Not yet recruiting |
Pain, Postoperative|Surgery--Complications|Opioid Analgesic Adverse Reaction|Opioid Use |
NCT04259333 |
| Cisplatin/Etoposide and Concurrent Radiotherapy With or Without Celecoxib in Patients With Unresectable Locally Advanced Non-small Cell Lung Cancer (NSCLC) |
Unknown status |
Lung Cancer |
NCT01503385 |
| Celecoxib in Decreasing the Damaging Effects of Sunburn in Healthy Volunteers |
Completed |
No Evidence of Disease |
NCT02090933 |
| Ciprofloxacin/Celecoxib Combination in Patients With ALS |
Recruiting |
ALS (Amyotrophic Lateral Sclerosis) |
NCT04090684 |
| Celecoxib With Chemotherapy in Localized, Muscle-Invasive Bladder Cancer |
Recruiting |
Bladder Cancer |
NCT02885974 |
| Open Label Study to Evaluate Ciprofloxacin/Celecoxib Combination in Patients With ALS |
Active, not recruiting |
Amyotrophic Lateral Sclerosis|ALS |
NCT04165850 |
| Clinical Study to Evaluate Effect on QT/QTc Interval After Multiple Dose of Celecoxib |
Completed |
Qt Interval, Variation in |
NCT03822520 |
| Celecoxib as Adjuvant Therapy to Chemotherapy in Patients With Metastatic Colorectal Cancer |
Recruiting |
Colon Cancer Stage |
NCT03645187 |
| Impact of Celecoxib on Electrophysiological Property in Brain of Healthy Volunteer |
Completed |
Electrophysiologic Property of Brain |
NCT02711579 |
| Gemcitabine and Celecoxib Combination Therapy in Treating Patients With R0 Resection Pancreatic Cancer |
Recruiting |
Pancreatic Cancer|Chemotherapy Effect |
NCT03498326 |
| A Study Of Nasopharyngeal Carcinoma (NPC) Treated With Celecoxib And ZD1839 |
Completed |
Nasopharyngeal Carcinoma |
NCT00212108 |
| Evaluating Celecoxib Activity in Mycobacterium Tuberculosis: A Whole Blood Bactericidal Activity Study in Healthy Volunteers |
Completed |
Tuberculosis |
NCT02602509 |
| Capecitabine and Celecoxib in Patients With Solid Cancers That Have Been Previously Treated With Standard Therapies |
Terminated |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT01705106 |
| Celebrex Premedication in Teeth With Symptomatic Irreversible Pulpitis |
Unknown status |
Pulpitis |
NCT03339544 |
| Celecoxib for Pediatric Adenotonsillectomy |
Completed |
Tonsillectomy|Adenotonsillectomy|Pain, Postoperative |
NCT00849966 |
| The Standard Care Versus Celecoxib Outcome Trial |
Completed |
Osteoarthritis|Rheumatoid Arthritis |
NCT00447759 |
| Preventive Effect of Celecoxib on Sorafenib-related Hand Foot Syndrome, a Single Center, Randomized Controlled Clinical Trail |
Completed |
Hepatocellular Carcinoma |
NCT02961998 |
| Safety and Efficacy of Etoricoxib 30 mg Versus Celecoxib 200 mg in Korean Participants With Osteoarthritis |
Completed |
Osteoarthritis of the Knee |
NCT01554163 |
| A Trial Assessing the Outcome of Celecoxib Administration Versus Placebo Following Anterior Cruciate Ligament (ACL) Reconstruction |
Completed |
Pain |
NCT01186887 |
| Study of the Combination Carboplatin Plus Celecoxib in Heavily Pre-treated Recurrent Ovarian Cancer Patients |
Completed |
Ovarian Neoplasms |
NCT01124435 |
| Pre-Prostatectomy Celecoxib or Placebo |
Terminated |
Adenocarcinoma of the Prostate|Prostate Cancer |
NCT02840162 |
| Celecoxib for Reducing Morphine Requirement After Thyroid Surgery: A Randomized Controlled Trial |
Completed |
Postoperative Pain|Thyroidectomy|Cyclooxygenase 2 Inhibitors |
NCT00520338 |
| Effectiveness of Celecoxib After Surgical Sperm Retrieval |
Terminated |
Pain |
NCT01323595 |
| Study of Celebrex (Celecoxib) in Patients With Recurrent Respiratory Papillomatosis |
Completed |
Recurrent Respiratory Papillomatosis |
NCT00571701 |
| Erlotinib, Celecoxib and Reirradiation for Recurrent Head and Neck Cancer |
Completed |
Cancer of the Pharynx|Cancer of the Larynx|Cancer of the Neck|Paranasal Sinus Neoplasms|Cancer of the Head |
NCT00970502 |
| The Effects of Celecoxib on Bone Ingrowth |
Terminated |
Bone Ingrowth|Pain |
NCT00585156 |
| Molecular Effects of Short-Term Celecoxib Treatment on Head and Neck Squamous Cell Carcinoma |
Completed |
SQUAMOUS CELL CARCINOMA |
NCT00596219 |
| Oral Tramadol Versus Oral Celecoxib for Post-perineal Repair Analgesia |
Not yet recruiting |
Perineal Pain |
NCT03694873 |
| A Study Of Celecoxib Versus Diclofenac In Patients With Ankylosing Spondylitis |
Completed |
Ankylosing Spondylitis |
NCT02528201 |
| Celecoxib and Docetaxel in Treating Patients With Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00030407 |
| Celebrex Total Knee Arthroplasty Study |
Terminated |
Osteoarthritis|Pain |
NCT00359151 |
| Celebrex - Cervix: Celecoxib in the Treatment of Patients With Locally Advanced Carcinoma of the Cervix |
Completed |
Cervix Neoplasms |
NCT00152828 |
| Celecoxib as Adjuvant Biologic Therapy in Patients With Head and Neck and Lung Cancer |
Terminated |
Head and Neck Cancer|Lung Cancer |
NCT00527982 |
| Celecoxib in Patients With Newly Diagnosed GBM Who Are Receiving Anticonvulsant Drugs and Undergoing RT |
Terminated |
Brain and Central Nervous System Tumors |
NCT00068770 |
| Celecoxib as a Post-tonsillectomy Pain Medication |
Completed |
Tonsillitis |
NCT00583453 |
| Trial of Celecoxib With Preoperative Chemo- Radiation for Locally Advanced Rectal Cancer |
Completed |
Locally Advanced Rectal Cancer |
NCT00931203 |
| Etanercept and Celecoxib Alone/Combined Treatment in Effectiveness and Safety of Active Ankylosing Spondylitis |
Completed |
Ankylosing Spondylitis |
NCT01934933 |
| Celecoxib and Docetaxel in Treating Patients With Advanced Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00030420 |
| A Double-blind and Randomized Trial of Celecoxib Added to Risperidone in Treatment-naive First-episode Schizophrenia |
Completed |
Schizophrenia |
NCT00686140 |
| Celecoxib After Tonsillectomy |
Completed |
Tonsillectomy |
NCT02934191 |
| Effect of Diacerein vs Celecoxib on Symptoms and Structural Changes in Symptomatic Knee Osteoarthritis |
Active, not recruiting |
Osteoarthritis|Osteoarthritis, Knee |
NCT02688400 |
| Study Of "Continuous Use" Of Celecoxib Vs. "Usual or Intermittent Use" |
Completed |
Osteoarthritis, Knee|Osteoarthritis, Hip |
NCT00139776 |
| Evaluation of Post-Procedure Administration of Celecoxib Following Shoulder Surgery |
Active, not recruiting |
Reverse or Primary Total Shoulder|Rotator Cuff- Full Thickness- Repair |
NCT03305068 |
| Celecoxib in Preventing Polyps in Patients Who Have Undergone Surgery for Stage I Colon Cancer |
Terminated |
Colorectal Cancer |
NCT00087256 |
| Effect of Celecoxib on Perioperative Inflammatory Response in Colon Cancer |
Terminated |
Colorectal Cancer|Colon Cancer |
NCT01284504 |
| Study to Compare the Efficacy and Safety of CELBESTA® and CELEBREX® in Patients With Rheumatoid Arthritis |
Completed |
Rheumatoid Arthritis |
NCT02780323 |
| Celecoxib Through Surgery and Radiation Therapy for the Treatment of Advanced Head and Neck Cancer |
Recruiting |
Clinical Stage III HPV-Mediated (p16-Positive) Oropharyngeal Carcinoma AJCC v8|Clinical Stage IV HPV-Mediated (p16-Positive) Oropharyngeal Carcinoma AJCC v8|Nasal Cavity and Paranasal Sinus Carcinoma|Oral Cavity Carcinoma|Pathologic Stage III HPV-Mediated (p16-Positive) Oropharyngeal Carcinoma AJCC v8|Pathologic Stage IV HPV-Mediated (p16-Positive) Oropharyngeal Carcinoma AJCC v8|Recurrent Hypopharyngeal Carcinoma|Recurrent Laryngeal Carcinoma|Recurrent Nasal Cavity and Paranasal Sinus Carcinoma|Recurrent Oral Cavity Carcinoma|Recurrent Oropharyngeal Carcinoma|Stage III Hypopharyngeal Carcinoma AJCC v8|Stage III Laryngeal Cancer AJCC v8|Stage III Oropharyngeal (p16-Negative) Carcinoma AJCC v8|Stage IV Hypopharyngeal Carcinoma AJCC v8|Stage IV Laryngeal Cancer AJCC v8|Stage IV Oropharyngeal (p16-Negative) Carcinoma AJCC v8|Stage IVA Hypopharyngeal Carcinoma AJCC v8|Stage IVA Laryngeal Cancer AJCC v8|Stage IVA Oropharyngeal (p16-Negative) Carcinoma AJCC v8|Stage IVB Hypopharyngeal Carcinoma AJCC v8|Stage IVB Laryngeal Cancer AJCC v8|Stage IVB Oropharyngeal (p16-Negative) Carcinoma AJCC v8|Stage IVC Hypopharyngeal Carcinoma AJCC v8|Stage IVC Laryngeal Cancer AJCC v8|Stage IVC Oropharyngeal (p16-Negative) Carcinoma AJCC v8 |
NCT04162873 |
| ATRA, Celecoxib, and Itraconazole as Maintenance |
Completed |
Relapsed Multiple Myeloma |
NCT02401295 |
| An Open-Label Study Of Celecoxib In Patients With Posttraumatic Pain |
Completed |
Pain |
NCT00976716 |
| Celecoxib as a Chemopreventive Agent in Current and Former Smokers |
Completed |
Smoking|Prevention |
NCT00981201 |
| Celecoxib in Preventing Cancer in Patients With Oral Leukoplakia and/or Head and Neck Dysplasia |
Completed |
Head and Neck Cancer |
NCT00052611 |
| Safety of Celecoxib in Patients With Crohn's Disease |
Terminated |
Crohn's Disease |
NCT00177866 |
| Celecoxib and Erlotinib in Treating Former Smokers With Stage IIIB or Stage IV Non-Small Cell Lung Cancer |
Completed |
Recurrent Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer |
NCT00088959 |
| The Effect of a Non-hormonal Cox-2 Inhibitor (Celebrex) on Ovulation |
Completed |
Ovulation|Luteal Development |
NCT01129245 |
| CPT-11/Cisplatin and Celecoxib With Radiation Therapy for Patients With Unresectable Non-Small Cell Lung Cancer (NSCLC) |
Completed |
Lung Cancer |
NCT00346801 |
| Safety of Fluvastatin-Celebrex Association in Low-grade and High Grade Optico-chiasmatic Gliomas |
Active, not recruiting |
Glioma |
NCT02115074 |
| Phase I Study to Evaluate Bioavailability of Overencapsulated Celecoxib |
Completed |
Healthy Volunteer |
NCT00729495 |
| Radiosensitization With Celecoxib and Chemoradiation for Head and Neck Cancer |
Completed |
Cancer |
NCT00581971 |
| Gefitinib and Celecoxib in Treating Patients With Refractory Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00068653 |
| Celecoxib to Prevent Colorectal Cancer in Patients Who Have Undergone Surgery to Remove Polyps |
Completed |
Colon Cancer|Rectal Cancer |
NCT00005094 |
| Delivra-Celecoxib 8% Cream and Osteoarthritis |
Terminated |
Osteoarthritis, Knee |
NCT03698916 |
| Celecoxib for the Treatment of Non-muscle Invasive Bladder Cancer |
Completed |
Non Muscle Invasive Bladder Cancer |
NCT02343614 |
| Exemestane With Celecoxib as Neoadjuvant Treatment in Postmenopausal Women With Stage II, III, and IV Breast Cancer |
Completed |
Breast Cancer |
NCT00201773 |
| Gemcitabine and Celecoxib in Treating Patients With Metastatic Pancreatic Cancer |
Completed |
Pancreatic Cancer |
NCT00068432 |
| Observational Familial Adenomatous Polyposis Registry Study In Patients Receiving Celecoxib Compared to Control Patients |
Terminated |
Familial Adenomatous Polyposis (FAP) |
NCT00151476 |
| Tramadol Versus Celecoxib for Reducing Pain in Outpatient Hysteroscopy |
Completed |
Pain |
NCT02071303 |
| Celecoxib/Oxaliplatin/Capecitabine for Gastric/Gastroesophageal Junction Carcinoma |
Terminated |
Gastric Carcinoma|Gastroesophageal Junction Carcinoma |
NCT00256321 |
| Celecoxib, Irinotecan and Concurrent Radiotherapy in Preoperative Pancreatic Cancer |
Terminated |
Pancreatic Cancer |
NCT00177853 |
| Administration of Celecoxib for Treatment of Intracerebral Hemorrhage : A Pilot Study |
Completed |
Intracerebral Hemorrhage |
NCT00526214 |
| Efficacy and Safety of Postoperative Intravenous Parecoxib Sodium Followed by Oral Celecoxib in Osteoarthritis Patients |
Completed |
Pain|Inflammation |
NCT02198924 |
| Celecoxib in Treating Patients With Early-Stage Rectal Cancer |
Terminated |
Colorectal Cancer |
NCT00608595 |
| Pregabalin, Celecoxib, Total Knee Arthroplasty and Intrathecal Morphine |
Completed |
Postoperative Pain Management|Total Knee Arthroplasty |
NCT01344213 |
| Efficacy of Celecoxib 200mg in Relieving Pain and Walking Dysfunction in Osteoarthritis of the Knee |
Unknown status |
Osteoarthritis of the Knee |
NCT00597415 |
| Open Label Comparative Study On Celecoxib Efficacy And Safety Vs Non-Selective NSAID In Acute Pain Due To Ankle Sprain |
Completed |
Ankle Sprain |
NCT00446797 |
| Study of CG100649 Versus Celecoxib in Osteoarthritis Patients |
Completed |
Osteoarthritis |
NCT01341405 |
| Celecoxib in Treating Patients With Progressive Metastatic Differentiated Thyroid Cancer |
Completed |
Head and Neck Cancer |
NCT00061906 |
| Celecoxib in Preventing Breast Cancer in At-Risk Premenopausal Women |
Completed |
Breast Cancer |
NCT00056082 |
| Tramadol Versus Celecoxib in Reducing Pain Associated With IUD Insertion |
Recruiting |
Pain |
NCT02827487 |
| Celecoxib in Preventing Non-Small Cell Lung Cancer in Tobacco Smokers |
Completed |
Lung Cancer |
NCT00020878 |
| Effects of Celecoxib After Percutaneous Coronary Intervention |
Completed |
Coronary Arteriosclerosis |
NCT00292721 |
| Lidocaine Patch 5% Versus Celecoxib 200 mg in Chronic Axial Low Back Pain |
Terminated |
Chronic Low Back Pain |
NCT00904397 |
| Celecoxib Compared With No Treatment Before Surgery in Treating Patients With Localized Prostate Cancer |
Completed |
Prostate Cancer |
NCT00022399 |
| Toradol v. Celecoxib for Postoperative Pain |
Completed |
Pain, Postoperative |
NCT03331315 |
| Lidocaine Patch Versus Celecoxib in Pain From Osteoarthritis of the Knee |
Terminated |
Osteoarthritis of the Knee |
NCT00904605 |
| Changes in Breast Cancer Biomarkers Using Synergistic Prostaglandin Inhibitors |
Completed |
Breast Cancer |
NCT01425476 |
| Efficacy and Safety Study of Celecoxib and Pregabalin Compared With Celecoxib Monotherapy, in Patients With Chronic Low Back Pain Having a Neuropathic Component |
Terminated |
Chronic Low Back Pain With a Neuropathic Component |
NCT01838044 |
| Celecoxib (Celebrex) Versus Placebo in Men With Recurrent Prostate Cancer |
Completed |
Prostate Cancer |
NCT00136487 |
| Celecoxib Versus Naproxen for Prevention of Recurrent Ulcer Bleeding in Arthritis Patients |
Completed |
Arthritis|Cardiovascular Diseases|Cerebrovascular Disorders |
NCT00153660 |
| Arthroplasty Inflammation Prophylaxis With Celecoxib |
Unknown status |
Postoperative Pain |
NCT00533247 |
| Evaluation of Celecoxib Effects on Amlodipine in Subjects With Existing Hypertension Requiring Antihypertensives |
Completed |
Hypertension |
NCT02979197 |
| A Phase II Trial of Combination Therapy With Celecoxib and Taxotere for the Treatment of Stage D3 Prostate Cancer |
Unknown status |
Prostate Cancer |
NCT00215345 |
| 99Tc-MDP Treatment for Knee Osteoarthritis |
Recruiting |
Treatment Outcome |
NCT02993029 |
| Celecoxib in Treating Patients With Stage I, Stage II, or Stage IIIA Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00653250 |
| Celecoxib for Primary Prophylaxis of Combat-Related Heterotopic Ossification |
Unknown status |
Ossification, Heterotopic |
NCT01631669 |
| Combination of Oxaliplatin, Capecitabine, and Celecoxib With Concurrent Radiation for Rectal Cancer |
Terminated |
Rectal Cancer |
NCT00250835 |
| Use of Celecoxib in Patients With Intraductal Papillary Mucinous Neoplasms (IPMNs) |
Terminated |
Pancreas Neoplasms |
NCT00198081 |
| Celecoxib in Preventing Multiple Myeloma in Patients With Monoclonal Gammopathy or Smoldering Myeloma |
Completed |
Monoclonal Gammopathy of Undetermined Significance|Multiple Myeloma|Smoldering Multiple Myeloma |
NCT00099047 |
| Study to Evaluate the Effect of Celecoxib on the Efficacy and Safety of Amlodipine in Subjects With Hypertension Requiring Antihypertensive Therapy |
Completed |
Hypertension |
NCT02172040 |
| Compare Celecoxib and Ibuprofen Sr In The Management Of Acute Pain Post Orthopedic Or Gynecological Surgery |
Completed |
Pain, Postoperative |
NCT00150280 |
| Naturalistic Safety Registry Of Celecoxib (CELEBREX(R)) And NSAIDs In Juvenile Idiopathic Arthritis |
Terminated |
Arthritis, Juvenile Rheumatoid |
NCT00688545 |
| Celecoxib in Treating Patients With Bladder Cancer |
Completed |
Recurrent Bladder Cancer |
NCT00006124 |
| Vitamin D Plus Celecoxib Therapy to Stimulate Intratumoral Immune Reactivity |
Completed |
Mouth Neoplasms |
NCT00953849 |
| Celecoxib (Celebrex) in the Management of Acute Renal Colic |
Withdrawn |
Ureteral Calculi |
NCT00304317 |
| Perioperative Pain Control With Celecoxib (Celebrex) in Total Knee Arthroplasty |
Completed |
Osteoarthritis |
NCT00598234 |
| A Pilot Study of Celecoxib in Patients With Grade 2 or 3 Uterine Cancers |
Terminated |
Uterine Cancer |
NCT00231829 |
| Celecoxib and Radiation Therapy in Treating Patients With Locally Advanced Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00046839 |
| Study on Efficacy and Safety of Chondroitin Sulfate + Glucosamine Hydrochloride Versus Celecoxib in Knee Osteoarthritis |
Completed |
Knee Osteoarthritis |
NCT01425853 |
| Doxorubicin and Strontium-89 With or Without Celecoxib in Treating Patients With Progressive Androgen-Independent Prostate Cancer and Bone Metastases |
Terminated |
Metastatic Cancer|Prostate Cancer |
NCT00080782 |
| Evaluation of Surgically Resected Colorectal Adenomas and Carcinomas After 7 Days Pretreatment With Celecoxib |
Completed |
Colorectal Adenoma|Colorectal Carcinoma |
NCT00582660 |
| The Pharmacokinetic Interaction Between Celecoxib and Rebamipide |
Completed |
Healthy Male Volunteers |
NCT01549743 |
| Secondary Primary Tumor Prevention With EGFR, OSI-774, and Cyclooxygenase-2 |
Completed |
Head and Neck Cancer |
NCT00400374 |
| Trial In Pediatric Patients With Familial Adenomatous Polyposis (FAP) |
Terminated |
Adenomatous Polyposis Coli |
NCT00585312 |
| Co-crystal E-58425 vs Tramadol and Celecoxib for Moderate to Severe Acute Pain After Bunionectomy. Phase III Clinical Trial. |
Completed |
Acute Post-surgical Pain |
NCT03108482 |
| Study of Celecoxib Bioavailability in Healthy Subjects |
Completed |
Healthy Volunteers |
NCT00296127 |
| N2012-01: Phase 1 Study of Difluoromethylornithine (DFMO) and Celecoxib With Cyclophosphamide/Topotecan |
Active, not recruiting |
Neuroblastoma |
NCT02030964 |
| Celecoxib in Preventing Colorectal Cancer in Young Patients With a Genetic Predisposition for Familial Adenomatous Polyposis |
Completed |
Colorectal Cancer|Precancerous Condition |
NCT00685568 |
| Tramadol Versus Celecoxib for Reducing Pain During Operative Office Hysteroscopy |
Unknown status |
Hysteroscopy |
NCT02736071 |
| A Study To Evaluate The Effects Of Celecoxib (Celebrex®) Or Naproxen On Blood Pressure In Pediatric Subjects |
Completed |
Arthritis, Juvenile Rheumatoid |
NCT00807846 |
| Effect of Celecoxib on Survival in Patients With Advanced Non-Small Cell Lung Cancer Receiving Chemotherapy |
Unknown status |
Non-Small Cell Lung Cancer |
NCT00300729 |
| Tramadol Versus Celecoxib for Reducing Pain During Office Hysteroscopy in Post Menopausal Women |
Unknown status |
Hysteroscopy |
NCT02736019 |
| CI(R)CA : Coumadin Interaction With Rofecoxib, Celecoxib and Acetaminophen |
Completed |
Antiphospholipid Antibody Syndrome |
NCT01762891 |
| Study To Evaluate Efficacy And Safety Of An Additional Dose Of Celecoxib (YM177) In Patients With Post-Tooth Extraction Pain |
Completed |
Pain |
NCT01062113 |
| Celecoxib Japan Observational Study for the Patients With Acute Pain |
Completed |
Patients With Traumatic Pain, Post-surgical Pain and Tooth Extract Pain |
NCT01876121 |
| Phase I/II Study of Celebrex and EPO906 in Patients With Metastatic Colorectal Cancer |
Unknown status |
Colon Cancer|Colorectal Cancer |
NCT00159484 |
| Erlotinib and Celecoxib in Treating Patients With Stage IIIB or Stage IV Recurrent Non-Small Cell Lung Cancer |
Completed |
Recurrent Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer |
NCT00062101 |
| Celecoxib in Preventing Lung Cancer in Former Heavy Smokers |
Completed |
Lung Cancer |
NCT00055978 |
| Celecoxib in Preventing Hand/Foot Syndrome Caused By Capecitabine With Metastatic Breast or Colorectal Cancer |
Terminated |
Breast Cancer|Colorectal Cancer|Pain |
NCT00305643 |
| Celecoxib in Treating Patients With Cervical Intraepithelial Neoplasia |
Completed |
Cervical Carcinoma|Cervical Intraepithelial Neoplasia Grade 2/3|Stage 0 Cervical Cancer |
NCT00081263 |
| Pharmacokinetics Of Celecoxib Test Formulations |
Completed |
Healthy Volunteers |
NCT00925106 |
| Evaluation of Post Hospital Administration of Celecoxib Following Minimally Invasive Knee Replacement Surgery |
Completed |
Pain Management|Function|Minimally Invasive Total Knee Arthroplasty|Celecoxib |
NCT00474773 |
| A Study of Epirubicin With Estramustine Phosphate and Celecoxib for the Treatment of Prostate Cancer |
Unknown status |
Prostate Cancer |
NCT00218205 |
| Celecoxib in Treating Postmenopausal Women Who Are Undergoing Surgery for Invasive Breast Cancer |
Completed |
Stage I Breast Cancer|Stage II Breast Cancer|Stage IIIA Breast Cancer|Stage IIIB Breast Cancer|Stage IIIC Breast Cancer |
NCT00070057 |
| Gastro-protective Effect and Pain Relief Effect of Naxozol Compared to Celecoxib in Patients With Osteoarthritis |
Unknown status |
Osteoarthritis |
NCT02355236 |
| Trial to Evaluate Efficacy and Safety of Combination of Diacerein and Celecoxib Administered in Patients With Knee OA |
Unknown status |
Knee Osteoarthritis |
NCT03404479 |
| Celecoxib Treatment for Lung Cancer |
Withdrawn |
Non-Small-Cell Lung Carcinoma |
NCT00108186 |
| Celecoxib in Treating Women With Metastatic or Recurrent Breast Cancer |
Terminated |
Breast Cancer |
NCT00045591 |
| Cyclophosphamide With or Without Celecoxib in Treating Patients With Recurrent or Persistent Ovarian Epithelial, Fallopian Tube, or Primary Peritoneal Cancer |
Active, not recruiting |
Fallopian Tube Cancer|Peritoneal Cavity Cancer|Recurrent Ovarian Epithelial Cancer |
NCT00538031 |
| Condrosulf vs Celebrex vs Placebo in the Treatment of Knee OA |
Completed |
Knee Osteoarthritis |
NCT02079727 |
| S0300, Celecoxib in Preventing Breast Cancer in Premenopausal Women |
Terminated |
Breast Cancer |
NCT00088972 |
| Effect Of Celecoxib On Hip Osteoarthritis (OA) Progression |
Terminated |
Osteoarthritis |
NCT00163241 |
| Celecoxib Efficacy And Safety Versus Diclofenac In Acute Pain Due To Cervical Sprain Related To A Traffic Accident |
Terminated |
Pain |
NCT00894790 |
| Study of Epicutaneously Applied Ketoprofen Transfersome® Gel With or Without Combination With Oral Celecoxib for the Treatment of Muscle Pain Induced by Eccentric Exercise |
Completed |
Musculoskeletal Pain |
NCT01020279 |
| A Study Of CP-195543 And Celecoxib Dual Therapy In Subjects With Rheumatoid Arthritis |
Terminated |
Arthritis, Rheumatoid |
NCT00424294 |
| Zileuton With or Without Celecoxib As Chemopreventive Agents in Smokers |
Completed |
Tobacco Use Disorder |
NCT01021215 |
| Celecoxib in Preventing the Damaging Effects of Sunburn in Healthy Volunteers |
Completed |
No Evidence of Disease |
NCT02099136 |
| Parecoxib Versus Celecoxib Versus Oxycodone in Pain Control for Transcatheter Chemoembolization Procedure |
Completed |
Liver Cancer|Pain Postoperative |
NCT03059238 |
| Polarized Dendritic Cell (aDC1) Vaccine, Interferon Alpha-2, Rintatolimod, and Celecoxib for the Treatment of HLA-A2+ Refractory Melanoma |
Not yet recruiting |
HLA-A2 Positive Cells Present|Refractory Melanoma |
NCT04093323 |
| A Study to Verify the Efficacy of YM177 (Celecoxib) in Postoperative Pain Patients |
Completed |
Post Operative Pain |
NCT01118572 |
| Timed Aspirin Chronobiome Study |
Not yet recruiting |
Healthy |
NCT03590821 |
| Celebrex With Preoperative Chemoradiation - Rectal Cancer |
Completed |
Colorectal Neoplasms |
NCT00188565 |
| Protocol for Women at Increased Risk of Developing Breast Cancer |
Completed |
Breast Cancer |
NCT00291694 |
| Celecoxib in Preventing Skin Cancer in Patients With Actinic Keratoses |
Withdrawn |
Precancerous Condition |
NCT00027976 |
| Vinorelbine and Celecoxib in Treating Women With Relapsed or Metastatic Breast Cancer |
Terminated |
Breast Cancer |
NCT00075673 |
| A Trial of Maintenance ADAPT Therapy With Capecitabine and Celecoxib in Patients With Metastatic Colorectal Cancer |
Terminated |
Recurrent Colon Carcinoma|Recurrent Rectal Carcinoma|Stage IVA Colon Cancer|Stage IVA Rectal Cancer|Stage IVB Colon Cancer|Stage IVB Rectal Cancer |
NCT01729923 |
| Celecoxib Versus Hyoscine Butyl-bromide in Reducing Pain Associated With IUD. |
Completed |
IUD Insertion Pain |
NCT03499743 |
| Adjuvant Oral Decitabine and Tetrahydrouridine With or Without Celecoxib in People Undergoing Pulmonary Metastasectomy |
Withdrawn |
Neoplasm Metastasis|Sarcoma|Neoplasms, Germ Cell and Embryonal|Melanoma |
NCT02839694 |
| Study Of Celecoxib In Healthy Subjects |
Completed |
Healthy Volunteers |
NCT00994461 |
| Changes in Biomarkers Using Prostaglandin Inhibitors |
Completed |
Biomarker Change Linked to Breast Cancer |
NCT01769625 |
| Minocycline and Celecoxib as Adjunctive Treatments of Bipolar Depression |
Completed |
Depression|Bipolar Disorder|Bipolar Depression|Mood Disorders |
NCT02703363 |
| A Six Week Study Of The Pain Relieving Effects Of Celecoxib 200 Mg Twice Daily Compared To Tramadol 50 Mg Four Times Daily In Patients With Chronic Low Back Pain |
Completed |
Low Back Pain |
NCT00662558 |
| Study Of Celecoxib Or Diclofenac For Efficacy and Safety In Chinese Patients With Ankylosing Spondylitis |
Completed |
Ankylosing Spondylitis |
NCT00762463 |
| Celebrex and Metformin for Postoperative Hepatocellular Carcinoma |
Recruiting |
Liver Cancer |
NCT03184493 |
| Paclitaxel and Carboplatin With or Without Celecoxib Before Surgery in Treating Patients With Stage IIIA Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00062179 |
| Celecoxib in Preventing Cancer in Patients at High Risk for Ovarian Epithelial Cancer Who Are Undergoing Prophylactic Oophorectomy |
Withdrawn |
brca1 Mutation Carrier|brca2 Mutation Carrier|Ovarian Cancer |
NCT00084370 |
| Effect of Celecoxib on Transitional Pain After Outpatient Surgery |
Withdrawn |
Pain |
NCT00664690 |
| Exemestane + Celecoxib vs Exemestane + Placebo in Metastatic Breast Cancer |
Completed |
Metastatic Breast Cancer |
NCT00525096 |
| Celecoxib in Treating Patients With Relapsed Prostate Cancer Following Radiation Therapy or Radical Prostatectomy |
Terminated |
Prostate Cancer |
NCT00073970 |
| Molecular Targeting of 15-Lipoxygenase-1 (15-LOX-1) for Apoptosis Induction in Human Colorectal Cancers |
Active, not recruiting |
Familial Adenomatous Polyposis |
NCT00503035 |
| Celebrex Low Dose ASA Study Examining the Incidence of Gastroduodenal Ulcers in a Healthy Population |
Completed |
Peptic Ulcers |
NCT00137033 |
| Efficacy & Safety of rAd-IFN Administered With Celecoxib & Gemcitabine in Patients With Malignant Pleural Mesothelioma |
Recruiting |
Malignant Pleural Mesothelioma |
NCT03710876 |
| Open-Label Study Of Exemestane With Or Without Celecoxib In Postmenopausal Women With ABC Having Progressed On Tamoxifen |
Completed |
Breast Neoplasms |
NCT00038103 |
| A Pilot Open-Label Crossover Bioavailability Study of Celecoxib in Healthy Volunteers |
Completed |
Healthy Volunteers |
NCT00813241 |
| The Effect of Celecoxib on the Antiplatelet Effect of Aspirin and Clopidogrel in Normal Volunteers |
Completed |
Healthy |
NCT00882388 |
| Gemcitabine, Cisplatin, and Celecoxib Treatment of Metastatic Pancreatic Cancer |
Completed |
Pancreatic Cancer |
NCT00176813 |
| Adjuvant Celecoxib in Completely Resected pN1-2 NSCLC Patients |
Suspended |
Non-Small Cell Lung Cancer |
NCT00211952 |
| Testing The Effectiveness Of Celecoxib In Patients With Painful Sore Throat |
Completed |
Pharyngitis |
NCT00402987 |
| Neoadjuvant Celecoxib and Capecitabine Combined With Pelvic Irradiation in Treating Patients With Stage II or Stage III Adenocarcinoma (Cancer) of the Rectum |
Terminated |
Colorectal Cancer |
NCT00081224 |
| Atorvastatin Calcium and Celecoxib in Treating Patients With Rising PSA Levels After Local Therapy for Prostate Cancer |
Completed |
Prostate Cancer |
NCT01220973 |
| Celecoxib in Preventing Head and Neck Cancer in Patients With Oral Leukoplakia |
Completed |
Head and Neck Cancer|Precancerous Condition |
NCT00101335 |
| A Study of Women With an Early Diagnosis of Breast Cancer, Taking Celecoxib Between the Biopsy and Lumpectomy/Mastectomy |
Completed |
Breast Cancer |
NCT00328432 |
| COLA: A Pilot Clinical Trial of COX-2 Inhibition in LAM and TSC |
Active, not recruiting |
Lymphangioleiomyomatosis (LAM) |
NCT02484664 |
| Celebrex vs Tramadol in the Treatment of Chronic Lower Back Pain. |
Completed |
Back Pain |
NCT00290901 |
| Cisplatin, CPT-11 and Celecoxib With Radiation Therapy and Surgery for Operable Esophageal Cancer |
Completed |
Esophageal Cancer |
NCT00137852 |
| Phase II Study of Celecoxib and Concurrent Radiotherapy in Stage II-III NSCLC |
Completed |
Non Small Cell Lung Carcinoma |
NCT00181532 |
| Celecoxib in Treating Patients With Precancerous Lesions of the Mouth |
Completed |
Head and Neck Cancer |
NCT00014404 |
| Window of Opportunity Study Targeting the Inflammatory Milieu |
Active, not recruiting |
Stage IA Breast Cancer|Stage IB Breast Cancer|Stage II Breast Cancer|Stage IIIA Breast Cancer|Stage IIIB Breast Cancer|Stage IIIC Breast Cancer|Stage IV Breast Cancer |
NCT01881048 |
| Evaluation of Superiority of Valsartan+Celecoxib+Metformin Over Metformin Alone in Type 2 Diabetes Patients |
Not yet recruiting |
Type 2 Diabetes|High Blood Pressure|Arthritis|Obesity |
NCT03686657 |
| Phase 3 Study of Pelubiprofen Tab. & Celebrex Cap. in Rheumatoid Arthritis Patients |
Completed |
Pelubiprofen|Celebrex|Rheumatoid Arthritis |
NCT01781702 |
| Celecoxib in Preventing Cancer in Patients With Rectal Polyps or Colorectal Neoplasia |
Completed |
Colorectal Cancer |
NCT00043043 |
| Prospective Randomized Evaluation Of Celecoxib Integrated Safety Vs Ibuprofen Or Naproxen |
Completed |
Arthritis, Rheumatoid |
NCT00346216 |
| Nevada |
Las Vegas |
United States|Clinical Research Advantage/Peter Philander |
Incline Village |
| Quebec |
Saskatoon |
Canada|Recherche Clinique Sigma |
Westmount |
| Celecoxib in Preventing Basal Cell Carcinoma in Patients With Basal Cell Nevus Syndrome |
Completed |
Non-melanomatous Skin Cancer |
NCT00023621 |
| Toripalimab With or Without Celecoxib as Neoadjuvant Therapy in Resectable dMMR/MSI-H Colorectal Cancer |
Recruiting |
Colorectal Cancer|Mismatch Repair-deficient (dMMR)|Microsatellite Instability-high (MSI-H)|Neoadjuvant Therapy |
NCT03926338 |
| Celebrex (Celecoxib) Treatment of Laryngeal Papilloma |
Terminated |
Laryngeal Papilloma |
NCT00592319 |
| Irinotecan, Cisplatin, and Radiation Therapy With or Without Celecoxib in Treating Patients With Stage II, Stage III, or Stage IV Esophageal Cancer |
Completed |
Esophageal Cancer |
NCT00520091 |
| Celecoxib and Docetaxel or Pemetrexed in Treating Patients With Advanced Recurrent Non-Small Cell Lung Cancer |
Terminated |
Lung Cancer |
NCT00520845 |
| COX-2 Inhibitor With Concurrent Chemoradiation in Locally Advanced Head & Neck Carcinoma |
Unknown status |
Head and Neck Cancer |
NCT00603759 |
| Study on the Neoadjuvant Use of Chemotherapy and Celecoxib Therapy in Patients With Invasive Breast Cancer |
Completed |
Breast Cancer |
NCT00135018 |
| Preoperative Analgesia in Non-gynecological Cancerous Women Who Underwent Elective Total Abdominal Hysterectomy |
Recruiting |
Total Abdominal Hysterectomy ,Pain , Acute Postoperative,Gabapentin , Celecoxib |
NCT03672162 |
| GI-Reasons- A Trial Of GI Safety Of Celecoxib Compared With Non-Selective Nonsteroidal Antiinflammatory Drugs (NSAIDS) |
Completed |
Osteoarthritis |
NCT00373685 |
| North Carolina |
Shelby |
United States|Pfizer Investigational Site |
Raleigh |
| An Examination of Predictors of Indicators of Response to Celecoxib in Women Who Have a Diagnosis of Early Breast Cancer |
Completed |
Breast Cancer |
NCT00291122 |
| Effect of Celecoxib on Markers of Vascular Inflammation |
Completed |
Hypertension and Coronary Artery Disease |
NCT00471341 |
| Tumor Cell Vaccines With ISCOMATRIX Adjuvant and Celecoxib in Patients Undergoing Resection of Lung and Esophageal Cancers and Malignant Pleural Mesotheliomas |
Terminated |
Mesolthelioma|Esophageal Cancer|Lung Cancer|Thoracic Sarcomas|Thymoma |
NCT01258868 |
| Effect of Selective COX-2 Inhibition on Ulcer Healing |
Completed |
Arthritis|Gastric Ulcer |
NCT00153673 |
| Efficacy of Lumiracoxib in Relieving Moderate to Severe Post-dental Surgery Pain, Compared to Both Placebo and Celecoxib |
Completed |
Pain |
NCT00348491 |
| Oxaliplatin, Leucovorin Calcium, and Fluorouracil With or Without Celecoxib in Treating Patients With Stage III Colon Cancer Previously Treated With Surgery |
Active, not recruiting |
Colorectal Cancer |
NCT01150045 |
| Nebraska |
Kearney |
United States|Nebraska Cancer Research Center |
Kearney |
| Biomarkers in Women Receiving Chemotherapy and Celecoxib for Stage II or Stage III Breast Cancer That Can Be Removed by Surgery |
Terminated |
Breast Cancer |
NCT00665457 |
| Celecoxib and Recombinant Interferon Alfa-2b in Metastatic Kidney Cancer Who Have Undergone Surgery |
Completed |
Renal Cell Cancer|Stage IV Renal Cell Cancer |
NCT01158534 |
| Efficacy and Safety of Celecoxib Versus Ibuprofen in the Treatment of Osteoarthritis of the Knee (United States) |
Completed |
Osteoarthritis, Knee |
NCT00620867 |
| Pilot Study of Allogeneic Tumor Cell Vaccine With Metronomic Oral Cyclophosphamide and Celecoxib in Patients Undergoing Resection of Lung and Esophageal Cancers, Thymic Neoplasms, and Malignant Pleural Mesotheliomas |
Terminated |
Lung Cancer|Esophageal Cancer|Malignant Pleural Mesothelioma|Sarcoma|Thymic Carcinoma |
NCT01143545 |
| Celecoxib in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00104767 |
| A Trial of Thalidomide, Celecoxib, Etoposide and Cyclophosphamide in Patients With Relapsed or Progressive Cancer |
Completed |
Neoplasms |
NCT00165451 |
| Bortezomib and Celecoxib in Treating Patients With Advanced Solid Tumors |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00290680 |
| Celecoxib, Recombinant Interferon Alfa-2b, and Rintatolimod in Treating Patients With Colorectal Cancer Metastatic to the Liver |
Recruiting |
Metastatic Carcinoma in the Liver|Recurrent Colorectal Carcinoma|Stage IV Colorectal Cancer AJCC v7|Stage IVA Colorectal Cancer AJCC v7|Stage IVB Colorectal Cancer AJCC v7 |
NCT03403634 |
| The Effect of COX-2 Inhibitor on Radiosensitivity in Nasopharyngeal Carcinoma |
Unknown status |
Nasopharyngeal Carcinoma |
NCT02537925 |
| Efficacy and Safety of Celecoxib as Add-on Therapy to Risperidone Versus Risperidone Alone in Patients With Schizophrenia |
Completed |
Schizophrenia |
NCT00639483 |
| Cyclooxygenase-2 Inhibitor for Adjuvant Anticancer Effect in Patients With Biliary-pancreas Cancer |
Unknown status |
Bile Duct Cancer|Pancreatic Cancer |
NCT01111591 |
| Safety And Efficacy Of Celecoxib Versus Sodium Diclofenac In The Treatment Of Acute Low Back Pain |
Completed |
Low Back Pain |
NCT00640432 |
| Celecoxib and Erlotinib in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00072072 |
| Celecoxib in Treating Patients With Early-Stage Head and Neck Cancer or Non-Small Cell Lung Cancer |
Completed |
Head and Neck Cancer|Lung Cancer |
NCT00058006 |
| A Study of Irinotecan, Cisplatin and Celebrex in Patients With Metastatic or Unresectable Esophageal Cancer |
Terminated |
Esophageal Cancer |
NCT00183807 |
| Cetuximab & Celecoxib for Metastatic Colorectal Cancer or Colorectal Cancer That Cannot Be Removed by Surgery |
Completed |
Colorectal Cancer |
NCT00466505 |
| Celecoxib, Ibuprofen and the Antiplatelet Effect of Aspirin |
Completed |
Ischemic Heart Disease|Osteoarthritis |
NCT00565500 |
| Epirubicin and Celecoxib in Treating Patients With Hepatocellular Carcinoma |
Completed |
Liver Cancer |
NCT00057980 |
| Irinotecan and Celecoxib in Treating Patients With Unresectable or Metastatic Colorectal Cancer |
Completed |
Colorectal Cancer|Diarrhea |
NCT00084721 |
| Safety and Efficacy of Celecoxib Versus Diclofenac in the Treatment of Ankylosing Spondylitis |
Completed |
Spondylitis, Ankylosing |
NCT00648141 |
| Letrozole and Celecoxib in Treating Postmenopausal Women With Locally Advanced or Metastatic Breast Cancer |
Terminated |
Breast Cancer |
NCT00101062 |
| Celecoxib and Trastuzumab in Treating Women With Metastatic Breast Cancer |
Completed |
Breast Cancer |
NCT00006381 |
| Celecoxib Versus Diclofenac In The Treatment Of Osteoarthritis Of The Hip |
Completed |
Osteoarthritis, Hip |
NCT00174317 |
| Celecoxib, Paclitaxel, and Carboplatin in Treating Patients With Cancer of the Esophagus |
Completed |
Esophageal Cancer |
NCT00066716 |
| Study of the Effect of Chondroitin Sulfate on Structural Changes in Knee Osteoarthritis Patients Assessed by MRI |
Completed |
Knee Osteoarthritis |
NCT01354145 |
| Safety and Efficacy of Celecoxib Versus Placebo in the Treatment of Knee Osteoarthritis in Patients Who Were Unresponsive to Naproxen and Ibuprofen |
Completed |
Osteoarthritis, Knee |
NCT00638807 |
| Prevention of Colorectal Sporadic Adenomatous Polyps (PRESAP) |
Completed |
Colorectal Adenoma |
NCT00141193 |
| Efficacy and Safety of Celecoxib Versus Placebo in the Treatment of Patients With Osteoarthritis of the Knee Who Were Unresponsive to Naproxen and Ibuprofen |
Completed |
Osteoarthritis, Knee |
NCT00640627 |
| 6-TG, Capecitabine and Celecoxib Plus TMZ or CCNU for Anaplastic Glioma Patients |
Completed |
Anaplastic Glioma of Brain|Glioblastoma Multiforme|Brain Cancer |
NCT00504660 |
| Celecoxib and Radiation Therapy in Treating Patients With Stage II or Stage III Soft Tissue Sarcoma of the Arm, Hand, Leg, or Foot That Has Been Removed by Surgery |
Completed |
Childhood Malignant Fibrous Histiocytoma of Bone|Sarcoma |
NCT00450736 |
| Erlotinib Hydrochloride With or Without Celecoxib in Treating Patients With Stage IIIB-IV Non-Small Cell Lung Cancer |
Completed |
Recurrent Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer |
NCT00499655 |
| Cyclophosphamide and Celecoxib in Treating Patients With Advanced Cancer |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00551889 |
| Paclitaxel and Celecoxib in Treating Patients With Recurrent or Persistent Platinum-Resistant Ovarian Epithelial or Primary Peritoneal Cancer |
Terminated |
Ovarian Cancer|Primary Peritoneal Cavity Cancer |
NCT00084448 |
| Celecoxib and Erlotinib in Treating Patients With Liver Cancer |
Withdrawn |
Liver Cancer |
NCT00293436 |
| 52 Week, International, Multi-center, Randomized, Double-blind, Double-dummy, Parallel-group Clinical Trial to Compare Retention on Treatment, Safety, Tolerability & Efficacy of Lumiracoxib 100 mg od, Lumiracoxib 100 mg Bid & Celecoxib 200 mg od in Pts With Primary OA of Hip, Knee, Hand or Spine |
Completed |
Osteoarthritis |
NCT00145301 |
| Effects of Celecoxib On Restenosis After Coronary Intervention and Evolution of Atherosclerosis Trial |
Unknown status |
Angioplasty, Transluminal, Percutaneous Coronary|Coronary Restenosis |
NCT00500279 |
| Study Of Celecoxib Or Diclofenac And Omeprazole For Gastrointestinal (GI) Safety In High GI Risk Patients With Arthritis |
Completed |
Osteoarthritis|Arthritis, Rheumatoid |
NCT00141102 |
| Low Dose Aspirin Inhibition of COX-2 Derived PGE2 in Male Smokers |
Completed |
Tobacco Use Disorder|Smoking |
NCT01796951 |
| A Study to Evaluate the Efficacy and Safety of Celecoxib Suspension Compared to Naproxen Suspension in Patients With JRA |
Completed |
Arthritis, Juvenile Rheumatoid |
NCT00652925 |
| Efficacy of Celecoxib Vs Placebo to Prevent Pain in a Paced Walk |
Unknown status |
Knee Osteoarthritis |
NCT00194090 |
| Safety and Activity of F14 for Management of Pain Following Total Knee Replacement |
Active, not recruiting |
Total Knee Replacement|Postoperative Pain |
NCT03541655 |
| Safety and Efficacy of Celecoxib Versus Naproxen in the 6-Month Treatment of Knee Osteoarthritis |
Completed |
Osteoarthritis, Knee |
NCT00643799 |
| A Study of the Patterns of Use of Etoricoxib in France (MK-0663-148) |
Completed |
Osteoarthritis |
NCT01572675 |
| Temozolomide Alone or in Combination With Thalidomide and/or Isotretinoin and/or Celecoxib in Treating Patients Who Have Undergone Radiation Therapy for Glioblastoma Multiforme |
Completed |
Brain and Central Nervous System Tumors |
NCT00112502 |
| Adjuvant Tumor Lysate Vaccine and Iscomatrix With or Without Metronomic Oral Cyclophosphamide and Celecoxib in Patients With Malignancies Involving Lungs, Esophagus, Pleura, or Mediastinum |
Suspended |
Thoracic Sarcomas|Thorasic Cancers|Cancers of Non-thoracic Origin With Metastases to the Lungs or Pleura|Sarcoma|Melanoma |
NCT02054104 |
| Effect of Celecoxib Versus Placebo Before and After Knee Surgery on the Overall Use of Analgesics After Surgery |
Completed |
Arthroscopy |
NCT00633386 |
| Efficacy and Safety of Celecoxib Versus Ibuprofen in the Treatment of Osteoarthritis of the Knee (Europe) |
Completed |
Osteoarthritis, Knee |
NCT00630929 |
| Celecoxib, Capecitabine, and Irinotecan in Treating Patients With Recurrent or Metastatic Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00258232 |
| Phase I/II Study of Chemoprevention With EGFR and COX-2 Inhibitor |
Completed |
Precancerous Conditions |
NCT00314262 |
| Effect of Celecoxib Versus Placebo Before and After Knee Surgery on Overall Use of Analgesics After Surgery |
Completed |
Arthroscopy |
NCT00633438 |
| Phase II Trial of Docetaxel and Celecoxib in Patients With Androgen Independent Prostate Cancer |
Terminated |
Metastatic Prostate Cancer |
NCT00494338 |
| Effect of Naproxen, Aspirin, Celecoxib, or Clopidogrel on the Healing of Stomach and Intestinal Ulcers |
Completed |
Gastroduodenal Ulcer |
NCT00778193 |
| ANGIOCOMB Antiangiogenic Therapy for Pediatric Patients With Diffuse Brain Stem and Thalamic Tumors |
Completed |
Brainstem Glioma |
NCT01756989 |
| Study Evaluating the Effect on Gastroduodenal Mucosa of PA32540, PA32540 and Celecoxib, and Aspirin With Celecoxib |
Completed |
Healthy |
NCT00700687 |
| Combination Chemotherapy Treatments in Patients With Metastatic Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00230399 |
| Celecoxib With or Without Eflornithine in Preventing Colorectal Cancer in Patients With Familial Adenomatous Polyposis |
Terminated |
Colorectal Cancer|Familial Adenomatous Polyposis |
NCT00033371 |
| Comparison Of Celecoxib 200 Mg Bid, Loxoprofen Sodium 60 Mg Tid And Placebo In Low Back Pain In Japan |
Completed |
Low Back Pain |
NCT00141154 |
| Comparison of Small Bowel Lesions Associated With Celecoxib Versus Ibuprofen Plus Omeprazole |
Completed |
Bowel Diseases, Inflammatory |
NCT00640809 |
| MUA to Treat Postoperative Stiffness After Total Knee Arthroplasty |
Recruiting |
Stiffness Following Total Knee Arthroplasty |
NCT02739035 |
| Etoposide, Cyclophosphamide, Thalidomide, Celecoxib, and Fenofibrate in Relapsed or Progressive Cancer |
Completed |
Central Nervous System Tumor, Pediatric|Leukemia|Lymphoma|Neuroblastoma|Sarcoma|Unspecified Childhood Solid Tumor, Protocol Specific |
NCT00357500 |
| Phase I Study of Gene Induction Mediated by Sequential Decitabine/Depsipeptide Infusion With or Without Concurrent Celecoxib in Subjects With Pulmonary and Pleural Malignancies |
Completed |
Advanced Esophageal Cancers|Primary Small Cell Lung Cancers|Non-Small-Cell Lung Carcinoma|Pleural Mesotheliomas|Cancers of Non-thoracic Origin With Metastases to the Lungs or Pleura |
NCT00037817 |
| Celebrex Short Versus Long Therapy In Osteoarthritis Of The Knee |
Completed |
Osteoarthritis,Knee |
NCT00137410 |
| Does Optimal Control of Pre-operative Chronic and Acute Pain Predict Improved Function After Orthopedic Surgery? |
Completed |
Osteoarthritis |
NCT00581685 |
| Celecoxib or Observation After Radiation Therapy and Chemotherapy in Treating Patients With Stage II or Stage III Non-Small Cell Lung Cancer |
Unknown status |
Lung Cancer |
NCT00274898 |
| Combination Chemotherapy and Celecoxib in Treating Patients With Advanced Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00073866 |
| Phase I-II Multiple-Dose Safety and Efficacy Study of a Selective Inhibitor of Cyclooxygenase - 2 (SC-58635) in Hereditary Non-Polyposis Colorectal Cancer (HNPCC) Patients and Carriers |
Completed |
Colorectal Neoplasm|Hereditary Nonpolyposis |
NCT00001693 |
| Etoposide and Celecoxib in Patients With Advanced Cancer |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00551005 |
| Celecoxib, Leucovorin, Fluorouracil, and Oxaliplatin in Treating Patients With Metastatic Colorectal Cancer |
Unknown status |
Colorectal Cancer |
NCT00072553 |
| Celecoxib in Managing Pain, Weight Loss, and Weakness in Patients With Advanced Cancer |
Withdrawn |
Cachexia|Lymphoma|Melanoma (Skin)|Ovarian Cancer|Pain|Sarcoma|Unspecified Adult Solid Tumor, Protocol Specific |
NCT00093678 |
| Intensive Locoregional Chemoimmunotherapy for Recurrent Ovarian Cancer Plus Intranodal DC Vaccines |
Recruiting |
Cancer of Ovary|Cancer of the Ovary|Neoplasms, Ovarian|Ovarian Cancer|Ovary Cancer|Ovary Neoplasms |
NCT02432378 |
| Vaccine Therapy and Celecoxib in Treating Patients With Metastatic Nasopharyngeal Cancer |
Unknown status |
Head and Neck Cancer |
NCT00589186 |
| Celecoxib Combined With Fluorouracil and Leucovorin in Treating Patients With Resected Stage III Adenocarcinoma (Cancer) of the Colon |
Completed |
Colorectal Cancer |
NCT00085163 |
| NSAIDs Added to Anti-TNF Therapy Versus Anti-TNF Therapy Alone on Progression of Structural Damage in Ankylosing Spondylitis |
Active, not recruiting |
Ankylosing Spondylitis |
NCT02758782 |
| Celecoxib in Treating Patients With Head and Neck Cancer That Can Be Removed By Surgery |
Unknown status |
Head and Neck Cancer |
NCT00357617 |
| Delivra-Celecoxib 8% Cream and Osteoarthritis |
Recruiting |
Osteoarthritis, Knee |
NCT03882749 |
| Celecoxib, Fluorouracil, and Radiation Therapy in Treating Patients With Stage II or Stage III Rectal Cancer That Can Be Removed By Surgery |
Completed |
Colorectal Cancer |
NCT00336960 |
| Phase III Study of CG100649 in Osteoarthritis Patients |
Unknown status |
Localized Primary Osteoarthritis of Hip|Localized Primary Osteoarthritis of Knee |
NCT01765296 |
| Celecoxib and Rosiglitazone in Treating Patients Who Are Undergoing Cystoscopic Surveillance for Early-Stage Noninvasive Carcinoma of the Bladder or Radical Cystectomy for Muscle-Invasive Carcinoma of the Bladder |
Withdrawn |
Bladder Cancer |
NCT00084578 |
| Celecoxib to Treat Macular Degeneration in Patients Receiving Photodynamic Therapy |
Completed |
Macular Degeneration |
NCT00043680 |
| Treatment of Severe Influenza A Infection |
Completed |
Influenza, Human |
NCT02108366 |
| Photodynamic Therapy-Induced Immune Modulation: Part III |
Recruiting |
Actinic Keratosis|Photodynamic Therapy |
NCT03643744 |
| A Clinical Trial of YH23537 in Patients With Knee Osteoarthritis |
Completed |
Knee Osteoarthritis |
NCT02759198 |
| Preemptive Analgesia in Total Knee Arthroplasty |
Completed |
Acute Pain |
NCT03523832 |
| Celecoxib in Preventing Skin Cancer |
Withdrawn |
Non-melanomatous Skin Cancer |
NCT00025051 |
| A Comparison of Safety and Treatment in Subjects With Osteoarthritis Taking Low Dose Aspirin |
Completed |
Osteoarthritis|Peptic Ulcer |
NCT00175032 |
| Effects of Nonsteroidal Anti-inflammatory Drug (NSAID) on Inflammatory Lesion of Axial Spondyloarthritis |
Completed |
Spondylarthropathies|Magnetic Resonance Imaging |
NCT03190603 |
| Celecoxib to Prevent Cancer in Patients With Barrett's Esophagus |
Completed |
Esophageal Cancer |
NCT00005878 |
| Perioperative Use of a Selective COX2 Inhibitor in Patients Undergoing Elective Colorectal Surgery: the Effect on Post-operative Bowel Motility and Post-operative Pain |
Unknown status |
Prevention of Post Operative Ileus |
NCT02790203 |
| Preemptive Analgesia in Cruciate Reconstruction |
Completed |
Anterior Cruciate Ligament Injury |
NCT01017380 |
| The Role of COX-2 Inhibition in Salt Sensitivity of Blood Pressure |
Completed |
Hypertension |
NCT00624559 |
| A Clinical Trial Using Metronomic Oral Low-dose Cyclophosphamide Alternating With Low-dose Oral Methotrexate With Continuous Celecoxib and Weekly Vinblastine in Children and Adolescents With Relapsed or Progressing soliD Tumours. |
Unknown status |
Solid Tumours. |
NCT01285817 |
| Temozolomide, Thalidomide, and Celecoxib Following Radiation Therapy in Treating Patients With Newly Diagnosed Glioblastoma Multiforme |
Completed |
Brain and Central Nervous System Tumors |
NCT00047294 |
| Thalidomide, Celecoxib, and Combination Chemotherapy in Treating Patients With Relapsed or Refractory Malignant Glioma |
Completed |
Brain and Central Nervous System Tumors |
NCT00047281 |
| Radiation Therapy Plus Celecoxib, Fluorouracil, and Cisplatin in Patients With Locally Advanced Cervical Cancer |
Completed |
Cervical Cancer |
NCT00023660 |
| Maintenance Treatment for Ovarian Carcinoma in Remission by an Antiangiogenic Treatment Strategy |
Terminated |
Ovarian Carcinoma |
NCT01175772 |
| S0212 Celecoxib in Treating Patients With High-Grade Squamous Intraepithelial Lesions of the Cervix |
Withdrawn |
Stage 0 Cervical Cancer|High-grade Squamous Intraepithelial Lesion |
NCT00072540 |
| Combination Chemotherapy With or Without Celecoxib in Treating Patients With Metastatic Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00064181 |
| Efficacy Study of PN400 (VIMOVO) Twice Daily and Celebrex Once Daily in Patients With Osteoarthritis |
Completed |
Osteoarthritis |
NCT00665431 |
| Efficacy Study of PN400 (VIMOVO) Twice Daily and Celebrex Once Daily in Patients With Osteoarthritis |
Completed |
Osteoarthritis |
NCT00664560 |
| Phase III Trial of Gemcitabine, Curcumin and Celebrex in Patients With Metastatic Colon Cancer |
Unknown status |
Colon Neoplasm |
NCT00295035 |
| Celebrex In Acute Gouty Arthritis Study |
Completed |
Arthritis, Gouty |
NCT00549549 |
| Measuring Gait And Self-Reported Pain In Patients With Osteoarthritis Of The Knee Using Placebo/Oxycodone/Celecoxib. |
Terminated |
Osteoarthritis |
NCT00484718 |
| Cox-2 Inhibition in Radiation-induced Oral Mucositis |
Completed |
Oral Mucositis |
NCT00698204 |
| PROSTACOX : Metastatic Prostate Chemotherapy |
Completed |
Metastatic Prostate Cancer |
NCT00213694 |
| Transition From Acute to Chronic Back Pain : Effect of L-dopa,Gender,and Associated Brain Plasticity |
Withdrawn |
Chronic Back Pain |
NCT04082715 |
| Ketoprofen in Transfersome Compared to Oral Celecoxib and Placebo for Pain Associated With Osteoarthritis of the Knee |
Completed |
Osteoarthritis, Knee |
NCT00317733 |
| Clinical Trial of a COX-2 Inhibitor for the Treatment of Women With Preeclampsia |
Withdrawn |
Preeclampsia |
NCT00442676 |
| Efficacy and Safety of Lumiracoxib |
Completed |
Osteoarthritis |
NCT00267215 |
| Variability in Response to Non-steroidal Anti-inflammatory Drugs |
Active, not recruiting |
Healthy |
NCT02502006 |
| Chart Review: Unresectable/Metastatic Cholangiocarcinoma Treated With Irinotecan, Capecitabine and Celecoxib |
Completed |
Cholangiocarcinoma |
NCT00320918 |
| Study of Etanercept and Celecoxib to Treat Temporomandibular Disorders (Painful Joint Conditions) |
Completed |
Temporomandibular Joint Disorder |
NCT00001955 |
| Anti-inflammatory Agents and Cholesterol Metabolism |
Terminated |
Osteoarthritis |
NCT01279395 |
| Effect of Acupuncture to Endothelial Dysfunction Induced by Ischemia-reperfusion Injury |
Unknown status |
Healthy |
NCT02255006 |
| Combination Therapy Selection Trial in Amyotrophic Lateral Sclerosis |
Completed |
Amyotrophic Lateral Sclerosis |
NCT00355576 |
| Preventing Recurrent Ulcer Bleeding in Arthritis Patients Using Esomeprazole Plus Celecoxib |
Completed |
Arthritis|Bleeding Ulcer |
NCT00365313 |
| A Study Comparing the Effectiveness and Safety of Extended Release Tramadol HCl at 100 mg, 200 mg and 300 mg Doses to Placebo for the Treatment of Moderate to Severe Pain Due to Osteoarthritis (OA) |
Completed |
Chronic Pain |
NCT00348452 |
| Preemptive Analgesia Following Uterine Artery Embolization |
Terminated |
Uterine Fibroids|Uterine Artery Embolization |
NCT01555073 |
| Chemoprevention of Oral Premalignant Lesions |
Completed |
Precancerous Conditions |
NCT00036283 |
| Tumor Cell Vaccine for Patients Undergoing Surgery for Sarcomas, Melanomas, Germ Cell Tumors, or Malignancies That Have Metastasized to the Lungs, Pleura, or Mediastinum |
Terminated |
Sarcoma|Melanoma|Epithelial Malignancies|Pleural Malignancies |
NCT01313429 |
| Cyclooxygenase-2 (COX-2) Inhibitor Reduces Serum Prostatic Specific Antigen (PSA) Levels |
Completed |
Benign Prostatic Hyperplasia |
NCT01678313 |
| Quality of Life in Postmenopausal Women Who Are Receiving Either Exemestane or Anastrozole With or Without Celecoxib for Stage I, Stage II, or Stage IIIA Primary Breast Cancer |
Completed |
Breast Cancer |
NCT00090974 |
| Carboplatin and Gemcitabine Combined With Celecoxib and/or Zileuton in Treating Patients With Advanced Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00070486 |
| Celebrex for Pain Relief After Oral Surgery |
Completed |
Facial Pain |
NCT00006299 |
| A Study of Type-1 Polarized Dendritic Cell (αDC1) Vaccine in Combination With Tumor-Selective Chemokine Modulation (Interferon-α2b, Rintatolimod, and Celecoxib) in Subjects With Chemo-Refractory Metastatic Colorectal Cancer |
Withdrawn |
Metastatic Colorectal Cancer |
NCT02615574 |
| Evaluation of the Efficacy and Safety of Entelon Tab. 150mg in Patients With Osteoarthritis of Knee |
Completed |
Knee Osteoarthritis |
NCT01768520 |
| Correlation Between Acute Analgesia and Long-Term Function Following Ankle Injuries |
Unknown status |
Ligament Injury |
NCT02667730 |
| Gemcitabine Hydrochloride or Pemetrexed Disodium and Carboplatin With or Without Celecoxib in Treating Patients With Advanced Non-Small Cell Lung Cancer |
Terminated |
Lung Cancer |
NCT01041781 |
| NSAIDs vs. Coxibs in the Presence of Aspirin |
Recruiting |
Rheumatoid Arthritis|Cardiovascular Diseases |
NCT03699293 |
| Efficacy and Safety in a Randomised Acute Pain Study of MR308 (Tramadol/Celecoxib). |
Completed |
Acute Pain |
NCT02982161 |
| Vinblastine, Celecoxib, and Combination Chemotherapy in Treating Patients With Newly-Diagnosed Metastatic Ewing's Sarcoma Family of Tumors |
Completed |
Sarcoma |
NCT00061893 |
| Sirolimus in Combination With Metronomic Chemotherapy in Children With Recurrent and/or Refractory Solid and CNS Tumors |
Recruiting |
Cancer |
NCT02574728 |
| Prevention of Progression of Duodenal Adenomas in Patients With Familial Adenomatous Polyposis |
Completed |
Familial Adenomatous Polyposis|Duodenal Neoplasms|Duodenal Polyps |
NCT00808743 |
| GRC 27864 First in Man, Single Ascending Dose Study in Healthy Volunteers |
Completed |
Healthy |
NCT02179645 |
| Biomarkers of Neuroinflammation and Anti-Inflammatory Treatments in Major Depressive Disorder |
Completed |
Major Depressive Disorder |
NCT02362529 |
| Tumor Cell Vaccines and ISCOMATRIX With Chemotherapy After Tumor Removal |
Terminated |
Sarcoma|Melanoma|Epithelial Malignancies|Pleural Malignancy |
NCT01341496 |
| Exemestane and Celecoxib in Postmenopausal Women at High Risk for Breast Cancer |
Completed |
Breast Neoplasms |
NCT00073073 |
| Experimental Biomarker Study for Pain Thresholds |
Completed |
Pain |
NCT02695745 |
| Effectiveness and Safety of Different Doses of BI 1026706 in Patients With Postoperative Dental Pain |
Completed |
Pain, Postoperative |
NCT02084511 |
| Novel PET Radioligands as Inflammatory Biomarkers in Rheumatoid Arthritis and Myositis |
Recruiting |
Myositis |
NCT03912428 |
| αDC1 Vaccine + Chemokine Modulatory Regimen (CKM) as Adjuvant Treatment of Peritoneal Surface Malignancies |
Completed |
Malignant Neoplasm of Pancreas Metastatic to Peritoneal Surface|Malignant Peritoneal Mesothelioma|Peritoneal Carcinomatosis |
NCT02151448 |
| Anti-Inflammatory Treatment for Age-Associated Memory Impairment: A Double-Blind Placebo-Controlled Trial |
Completed |
Memory Disorders |
NCT00009230 |
| Does Duloxetine Reduce Chronic Pain After Total Knee Arthroplasty? |
Unknown status |
Neuropathic Pain |
NCT02307305 |
| Delaying Alzheimer Disease Symptoms With Anti-Inflammatory Drugs |
Completed |
Alzheimer Disease|Dementia |
NCT00065169 |
| NSAID Treatment in Knee Osteoarthritis |
Completed |
Knee Osteoarthritis |
NCT01860833 |
| Can DFN-15 Terminate Migraine With Allodynia? |
Completed |
Migraine With Aura|Migraine Without Aura|Allodynia |
NCT03472378 |
| Efficacy and Safety of Shinbaro Capsule |
Completed |
Osteoarthritis |
NCT01535417 |
| A Selective COX-2 Inhibitor Provides Pain Control But Hinders Healing Following Arthroscopic Rotator Cuff Repair |
Completed |
Rotator Cuff Tear |
NCT02850211 |
| Effect of COX-2 and EGFR Suppression on Molecular Markers of Angiogenesis and Proliferation in Squamous Cell Carcinoma of Oral Cavity - Prospective Randomized Study |
Active, not recruiting |
Oral Squamous Cell Carcinoma|Carcinoma of Buccal Mucosa|Tongue Cancers|Head and Neck Cancers |
NCT02748707 |
| A Multiple Dose Study of LY3023703 in Healthy Participants |
Completed |
Healthy Volunteers |
NCT01849055 |
| Efficacy and Safety of Lumiracoxib in Patients With Primary Knee Osteoarthritis(OA) |
Completed |
Osteoarthritis |
NCT00475800 |
| Maintenance Metronomic Chemotherapy for Metastatic Colorectal Carcinoma |
Terminated |
Colorectal Cancer Metastatic |
NCT01668680 |
| Treatment of Diabetic Nephropathy |
Terminated |
Diabetic Nephropathy |
NCT00065559 |
| A Study of MK0663 and an Approved Drug in the Treatment of Osteoarthritis (0663-077)(COMPLETED) |
Completed |
Osteoarthritis |
NCT00092781 |
| A Study of MK0663 and an Approved Drug in the Treatment of Osteoarthritis (0663-076)(COMPLETED) |
Completed |
Osteoarthritis |
NCT00092768 |
| Early Diuresis Following Colorectal Surgery |
Completed |
Colorectal Disorders |
NCT02351934 |
| Clinical Trial of Pregabalin and COX2 in Spinal Stenosis |
Not yet recruiting |
Spinal Stenosis Lumbar |
NCT03584074 |
| Phase III Trial of Gemcitabine, Curcumin and Celebrex in Patients With Advance or Inoperable Pancreatic Cancer |
Unknown status |
Pancreatic Cancer |
NCT00486460 |
| A Study of Two Approved Drugs in Patients With Osteoarthritis (0966-220)(COMPLETED) |
Completed |
Osteoarthritis |
NCT00092365 |
| A Study of Two Approved Drugs in Patients With Osteoarthritis (0966-219) |
Completed |
Osteoarthritis |
NCT00092352 |
| Active Drug Comparative, Multi-center, phase3 Clinical Study to Evaluate the Efficacy and Safety of PG201 in Osteoarthritis Patients |
Completed |
Osteoarthritis of the Knee |
NCT01576419 |
| Effects of Dual Cyclooxygenase-2 and Carbonic Anhydrase Inhibition |
Terminated |
Healthy Volunteers |
NCT00780325 |
| Nivolumab, Ipilimumab and COX2-inhibition in Early Stage Colon Cancer: an Unbiased Approach for Signals of Sensitivity |
Recruiting |
Colon Carcinoma |
NCT03026140 |
| Study Evaluating the Efficacy and Safety of Topical Diclofenac Spray in Osteoarthritis of the Knee: Trial II |
Completed |
Osteoarthritis |
NCT00546832 |
| Study Evaluating the Efficacy and Safety of Topical Diclofenac Spray in Osteoarthritis of the Knee: Trial I |
Completed |
Osteoarthritis |
NCT00546507 |
| Efficacy and Safety of Lumiracoxib in Patients With Knee Osteoarthritis (OA). |
Completed |
Osteoarthritis, Knee |
NCT00366938 |
| A Molecule Basic Study of Early Warning for New Pathogenic Risk of Ankylosing Spondylitis |
Completed |
Ankylosing Spondylitis |
NCT01709656 |
| Laser and Medical Treatment of Diabetic Macular Edema |
Completed |
Macular Degeneration |
NCT00050479 |
| Low Dose Chemotherapy Versus Best Supportive Care in Progressive Pediatric Malignancies |
Completed |
Malignant Childhood Neoplasm |
NCT01858571 |
| Efficacy and Safety of Lumiracoxib in Patients With Primary Knee Osteoarthritis (OA) |
Completed |
Knee Osteoarthritis |
NCT00367315 |
| Effects of Duloxetine on Pain Relief After Total Knee Arthroplasty in Central Sensitization Patient |
Unknown status |
Osteoarthritis |
NCT02600247 |
| Radial Extracorporeal Shock Wave Therapy for Chronic Non-specific Low Back Pain |
Unknown status |
Low Back Pain |
NCT03337607 |
| Late-Life Stress and Inflammation |
Suspended |
Depression|Inflammation |
NCT02389465 |
| Effectiveness of Two Different Doses of BI 1026706 on the Overall Peak-to-Peak (PtP) N2/P2-component Amplitude of Laser (Somatosensory, Radiant-heat) Evoked Potentials (LEP) in UVB(Ultraviolet)-Irradiated Skin in Healthy Male Volunteers |
Completed |
Healthy |
NCT02037165 |
| Efficacy and Safety of Lumiracoxib in Patients With Primary Knee Osteoarthritis (OA) |
Completed |
Osteoarthritis |
NCT00476034 |
| Chemokine Modulation Therapy and Standard Chemotherapy Before Surgery for the Treatment of Early Stage Triple Negative Breast Cancer |
Recruiting |
Anatomic Stage 0 Breast Cancer AJCC v8|Anatomic Stage I Breast Cancer AJCC v8|Anatomic Stage IA Breast Cancer AJCC v8|Anatomic Stage IB Breast Cancer AJCC v8|Anatomic Stage II Breast Cancer AJCC v8|Anatomic Stage IIA Breast Cancer AJCC v8|Anatomic Stage IIB Breast Cancer AJCC v8|Early-Stage Breast Carcinoma|Estrogen Receptor Negative|HER2/Neu Negative|Progesterone Receptor Negative|Prognostic Stage 0 Breast Cancer AJCC v8|Prognostic Stage I Breast Cancer AJCC v8|Prognostic Stage IA Breast Cancer AJCC v8|Prognostic Stage IB Breast Cancer AJCC v8|Prognostic Stage II Breast Cancer AJCC v8|Prognostic Stage IIA Breast Cancer AJCC v8|Prognostic Stage IIB Breast Cancer AJCC v8|Triple-Negative Breast Carcinoma |
NCT04081389 |
| Efficacy and Safety of Chuna Treatment as an Adjunctive Therapy After Knee Replacement |
Recruiting |
Total Knee Replacement|Manipulation |
NCT03625050 |
| Urinary NGF as A Biomarker for Acute Bacterial Cystitis |
Completed |
Urinary Tract Infection |
NCT01800799 |
| Phase I Study MK-3475 With Chemotherapy in Patients With Advanced GI Cancers |
Terminated |
Advanced GI Cancer |
NCT02268825 |
| For Prevention of Diarrhea in Patients Diagnosed With Metastatic Colorectal Cancer Treated With Chemotherapy |
Terminated |
Neoplasm Metastasis|Colorectal Neoplasms |
NCT00037180 |
| Long Term Safety and Efficacy Study of Tanezumab in Japanese Adult Subjects With Chronic Low Back Pain |
Completed |
Low Back Pain |
NCT02725411 |
| Efficacy and Safety Study of Lumiracoxib in Patients With Primary Hip Osteoarthritis |
Completed |
Osteoarthritis, Hip |
NCT00154219 |
| Clinical Research of the Prognostic Influence of NSAIDS's Anti-inflammatory Effect on Senior Patients With Hip Fracture |
Unknown status |
Hip Fracture |
NCT01583660 |
| A Study of LY3023703 Testing Pain Relief After Wisdom Teeth Removal |
Completed |
Acute Pain |
NCT01872910 |
| Effect of Selective and Nonselective Cyclooxygenase Enzyme Inhibition on Arterial Blood Pressure and Cerebral Blood Flow With Exposure to Intermittent Hypoxia in Humans |
Completed |
Obstructive Sleep Apnea|Hypertension|Cardiovascular Diseases|Stroke |
NCT01280006 |
| Preoperative Non-steroidal Anti-inflammatory Drugs(NSAID) to Colorectal Cancer Patients |
Completed |
Colorectal Cancer |
NCT00473980 |
| Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT) |
Completed |
Alzheimer Disease |
NCT00007189 |
| A Single Dose Study of LY3023703 in Healthy Participants |
Completed |
Healthy Volunteers |
NCT01632579 |
| Metronomic Treatment in Children and Adolescents With Recurrent or Progressive High Risk Neuroblastoma |
Recruiting |
Recurrent Neuroblastoma |
NCT02641314 |
| A Study of LY3031207 in Healthy Subjects |
Completed |
Healthy Volunteers |
NCT01449630 |
| Low Dose Metronomic Poly-chemotherapy for Metastatic CRC |
Completed |
Colorectal Cancer |
NCT02280694 |
| Anti-inflammatories and Adolescent Schizophrenia |
Recruiting |
Schizophrenia |
NCT04020588 |
| Comparing Postoperative Pain Following COX-2 and Prostanoids Expression |
Unknown status |
Female Genital Disease |
NCT03391570 |
| Chemokine-Modulatory Regimen for Recurrent Resectable Colorectal Cancer |
Terminated |
Colorectal Cancer|Colorectal Carcinoma|Colorectal Tumors|Neoplasms, Colorectal |
NCT01545141 |
| Dose Reduction of Etanercept in Patients With Ankylosing Spondylitis |
Unknown status |
Ankylosing Spondylitis |
NCT02638896 |
| Chemokine Modulation Therapy and Pembrolizumab in Treating Participants With Metastatic Triple-Negative Breast Cancer |
Recruiting |
Triple -Negative Breast Cancer|Estrogen Receptor Negative|HER2/Neu Negative|Anatomic Stage IV Breast Cancer AJCC|Progesterone Receptor Negative |
NCT03599453 |
| Efficacy, Tolerability, and Safety of DFN-15 |
Completed |
Migraine Headache |
NCT03009019 |
| Efficacy, Tolerability, and Safety Study of DFN-15 |
Completed |
Migraine Headache |
NCT03006276 |
| Low-Dose/Metronomic(LDM)Chemotherapy for Metastatic Breast Cancer |
Terminated |
Chemotherapy|Breast Cancer, Metastatic |
NCT01067989 |
| Trial Of Irinotecan In Combination With Three Methods Of Administration Of Fluoropyrimidine. |
Completed |
Colorectal Neoplasms |
NCT00101686 |
| A Pilot Study Assessing the Treatment Responsiveness of a Novel Osteoarthritis Stiffness Scale |
Completed |
Osteoarthritis, Knee |
NCT03570554 |
| The Clinical Efficacy of Non-steroidal Anti-inflammation Drugs in Patients With Benign Prostatic Hyperplasia |
Withdrawn |
Benign Prostatic Hyperplasia |
NCT00687388 |
| PECS Block vs. Multimodal Analgesia for Prevention of Persistent Postoperative Pain in Breast Surgery |
Active, not recruiting |
Breast Cancer|Breast Cancer Female|Cancer of Breast |
NCT03084536 |
| Statins and Selective Cyclooxygenase-2 Receptor Inhibitors in Blunt Chest Trauma |
Unknown status |
Blunt Chest Trauma|Acute Respiratory Distress Syndrome |
NCT01623921 |
| Clinical Trial of YYC301 for Treatment of Osteoarthritis of the Knee |
Not yet recruiting |
Osteoarthritis, Knee |
NCT03850587 |
| To Evaluate the Safety of 'Shinbaro Capsule'in Patients With Osteoarthritis |
Completed |
Osteoarthritis |
NCT01604239 |
| Single Dose Preoperative Gabapentin Use in Minimally Invasive Hysterectomy for Acute Pain Management |
Completed |
Pain, Postoperative |
NCT02703259 |
| A Study of LY3127760 in Healthy Participants |
Completed |
Healthy Volunteers |
NCT01968070 |
| Bipolar Depression and Inflammation |
Completed |
Bipolar Depression |
NCT01479829 |
| Tourniquet and Quadricepsforce |
Completed |
Osteoarthritis|Total Knee Arthroplasty |
NCT01808859 |
| Elderly Back Pain: Comparing Chiropractic to Medical Care |
Completed |
Subacute Low Back Pain|Chronic Low Back Pain |
NCT00602901 |
| A Study of High-Risk Oral Cavity Cancer |
Terminated |
Oral Cavity Cancer |
NCT00934739 |
| Improvement in Pain,Function and HRQoL ( Health Related Quality of Life) in Subacute Low Back Pain: A Controlled Clinical Trial of Exercise vs NSAIDs (Nonsteroidal Antiinflammatory) |
Completed |
Back Pain Lower Back |
NCT01374269 |
| Study to Determine the Safety and the Efficacy of Fasinumab Compared to Placebo and Nonsteroidal Anti-inflammatory Drugs (NSAIDs) for Treatment of Adults With Pain From Osteoarthritis of the Knee or Hip |
Active, not recruiting |
Osteoarthritis, Knee|Osteoarthritis, Hip |
NCT03304379 |
| Non-Steroidal Anti-inflammatory Drugs in Axial Spondyloarthritis |
Completed |
Ankylosing Spondylitis|Axial Spondyloarthritis |
NCT03473665 |
| Efficacy and Safety in a Randomised Acute Pain Study of MR308: STARDOM2. |
Completed |
Acute Pain |
NCT03062644 |
| The Effects of a Prostaglandin Inhibitor on Ovulation and the Menstrual Cycle |
Completed |
Ovulation (Follicular Rupture Yes/no)|Menstrual Cycles (Total Length, Bleeding Days)|Gonadotropin and Ovarian Hormone Levels (FSH, LH, E2, P) |
NCT00614406 |
| Study of Safety, Efficacy of Fulranumab Adjunctive Use in OA of Hip or Knee, PAI3007 |
Completed |
Osteoarthritis|Pain |
NCT02301234 |
| A Study of ARRY-371797 in Subjects Undergoing Third Molar Extraction |
Completed |
Dental Pain |
NCT00663767 |
| Efficacy of Multimodal Analgesia Following Hip Arthroscopy |
Recruiting |
Hip Pain Chronic |
NCT03351439 |
| Effect of Celecoxib and Etoricoxib on the Pharmacokinetics and Pharmacodynamics of Oral Tramadol |
Completed |
Healthy |
NCT01304069 |
| Pain Management in Head and Neck Surgery Patients |
Recruiting |
Pain Management |
NCT03121963 |
| A Multiple-dose Study of LY3031207 in Healthy Participants |
Terminated |
Healthy Volunteers |
NCT01632566 |
| Effect of COX-2 Selective Inhibitors on Postoperative Insulin Resistance After Gastrointestinal Laparoscopic Surgery |
Unknown status |
Gastrointestinal Cancer |
NCT01930318 |
| Additional Effect of Wound Infiltration After Cesarean Section With Optimal Standard Analgesia |
Completed |
Postoperative Pain|Anesthesia, Local|Breast Feeding |
NCT01751256 |
| An Efficacy, Safety and Effects on Quality of Life of Tramadol/Paracetamol as Add-on Therapy in Chronic Osteoarthritis |
Completed |
Osteoarthritis |
NCT01728246 |
| Medical Treatment of "High-Risk" Neurofibromas |
Unknown status |
Neurofibromatosis 1 |
NCT00846430 |
| A Study of LY2951742 in Participants With Mild to Moderate Osteoarthritis Knee Pain |
Terminated |
Osteoarthritis, Knee |
NCT02192190 |
| Study to Evaluate Arthroplasty Specimens for Osteoarthritis of the Knee and Hip |
Enrolling by invitation |
Osteoarthritis, Knee|Osteoarthritis, Hip |
NCT03949673 |
| Multimodal Analgesia in Shoulder Arthroplasty |
Recruiting |
Shoulder Pain|Opioid Use |
NCT03586934 |
| Follow-up Study of the Alzheimer's Disease Anti-Inflammatory Prevention Trial |
Unknown status |
Alzheimer Disease|Dementia |
NCT01417130 |
| The Effect of Parecoxib Sodium Intravenous Patient-controlled Analgesia in Laparotomic Liver Resection |
Unknown status |
Pain, Postoperative |
NCT02408146 |
| A Safety Trial to Compare Different Analgesics in Combination With Low Dose Aspirin to Study Their Bleeding Properties and Their Effects on the Stomach |
Completed |
Analgesics |
NCT00261586 |
| Biosynthesis of PGD2 in Vivo |
Completed |
Healthy Volunteer |
NCT01275300 |
| Study of Diclofenac Capsules to Treat Pain Following Bunionectomy |
Completed |
Other Acute Postoperative Pain |
NCT01462435 |
| Study on the Pharmacokinetics (PK) of DFN-15 vs Comparator and Food-effect on DFN-15 PK in Healthy Adult Subjects |
Completed |
Migraine |
NCT03282838 |
| Effects of Duloxetine on Postoperative Wound Complication of Total Knee Arthroplasty (TKA) in Central Sensitization Patients |
Not yet recruiting |
Osteoarthritis, Knee |
NCT03880916 |
| Gefitinib and Capecitabine in Treating Patients With Advanced Solid Tumors |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00039390 |
| Total Knee Arthroplasty (TKA) Study of HTX-011 in an Multimodal Analgesic Regimen (MMA) Regimen |
Recruiting |
Analgesia |
NCT03974932 |
| The Effect of Panax Notoginseng Powders on Rheumatic Pain |
Unknown status |
Rheumatic Pain |
NCT02260336 |
| Phase 2 Study of Indomethacin Capsules to Treat Dental Pain |
Completed |
Dental Pain |
NCT00964431 |
| A Feasibility Study of Multimodal Exercise/Nutrition/Anti-inflammatory Treatment for Cachexia - the Pre-MENAC Study |
Completed |
Cancer|Cachexia |
NCT01419145 |
| Study of Diclofenac Capsules to Treat Dental Pain |
Completed |
Dental Pain |
NCT00985439 |
| A Multimodal Analgesia Protocol Adapted for Ambulatory Surgery |
Recruiting |
Opioid Use |
NCT04015908 |
| Milgamma® and Milgamma® Compositum Step-therapy in Patients With Acute Non-specific Low Back Pain Receiving Modern NSAID |
Completed |
Acute Non-specific Low Back Pain |
NCT03892707 |
| Perioperative Analgesia on Postoperative Opioid Usage and Pain Control in H&N Cancer Surgery |
Recruiting |
Postoperative Pain Control|Opioid Consumption |
NCT04176419 |
| Dendritic Cell Based Therapy of Malignant Melanoma |
Completed |
Advanced Melanoma |
NCT00197912 |
| The Effect of Periarticular Multi-Drug Regimen on Pain After Partial Hip Replacement |
Unknown status |
Femoral Neck Fracture |
NCT01112436 |
| A Prospective Observational Study on Discontinuation of Medication of Shinbaro Capsule |
Unknown status |
Osteoarthritis |
NCT02064634 |
| Nonopioid Analgesia After Arthroscopic Meniscus Surgery |
Recruiting |
Meniscus Disorder|Narcotic Use |
NCT03820193 |
| Nonopioid Analgesia After Anterior Cruciate Ligament Reconstruction |
Recruiting |
Anterior Cruciate Ligament Injury |
NCT03818932 |
| Nonopioid Analgesia After Rotator Cuff Repair |
Recruiting |
Rotator Cuff Tear |
NCT03818919 |
| Nonopioid Analgesia After Labral Surgery |
Recruiting |
Narcotic Use |
NCT03825809 |
| Dextromethorphan Use in Multimodal Analgesia Regimens for Total Knee Arthroplasty |
Terminated |
Pain, Postoperative |
NCT02987920 |
| Different Kinds of Acupuncture Treatment for Knee Osteoarthritis |
Recruiting |
Knee Osteoarthritis |
NCT03563690 |
| Effect of a Multimodal Pain Regimen on Pain Control, Patient Satisfaction and Narcotic Use in Orthopaedic Trauma Patients |
Withdrawn |
Post Operative Pain Control |
NCT02160301 |
| A Personalized Medicine Study for Patients With Advanced Cancer of the Breast, Prostate, Pancreas or Those With Refractory Acute Myelogenous Leukemia |
Recruiting |
Breast Cancer|Prostate Cancer|Pancreatic Cancer|Acute Myelogenous Leukemia |
NCT03878524 |
| Evaluating Effectiveness of Educational Intervention to Help Physicians Address Inappropriate Patient Requests for Direct-to-Consumer Advertised Prescription Medications |
Completed |
Lumbosacral Muscle Strain |
NCT00915291 |
| Pre-induction Analgesia: Multimodel Regimen vs Aceteminophen for Post Ureteroscopy Pain |
Withdrawn |
Kidney Calculi|Pain, Postoperative |
NCT03549611 |
| Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT) |
Completed |
Osteoarthritis |
NCT00032890 |
| Study of Indomethacin Capsules to Treat Pain Following Bunionectomy |
Completed |
Other Acute Postoperative Pain |
NCT01543685 |
| Factors Affecting the Speed of Recovery After ACL Reconstruction |
Completed |
Anterior Cruciate Ligament Injury|ACL Injury|ACL - Anterior Cruciate Ligament Rupture |
NCT03770806 |
| Long Term Safety and Efficacy Study of Tanezumab in Subjects With Osteoarthritis of the Hip or Knee |
Completed |
Chronic Pain|Osteoarthritis, Hip|Osteoarthritis, Knee |
NCT02528188 |
| Croatia|National Hospital Organization Toyohashi Medical Center |
Aichi |
Funabashi |
Zagreb |
| Metronomic and Targeted Anti-angiogenesis Therapy for Children With Recurrent/Progressive Medulloblastoma |
Recruiting |
Medulloblastoma |
NCT01356290 |
| Short-term Prednisone to Treat STA Study(SPTSS) |
Completed |
Subacute Thyroiditis |
NCT01837433 |
| Safety and Efficacy of Two Dosages of Diractin® in Osteoarthritis (OA) |
Completed |
Osteoarthritis of the Knee |
NCT00716547 |
| Treatment of Orthostatic Hypotension in Autonomic Failure |
Completed |
Autonomic Failure|Orthostatic Hypotension |
NCT00223691 |
| Vaccine Therapy and Radiation to Liver Metastasis in Patients With CEA-Positive Solid Tumors |
Completed |
Liver Neoplasms |
NCT00081848 |
| Efficacy of Opioid-limiting Pain Management Protocol in Men Undergoing Urethroplasty |
Recruiting |
Pain, Postoperative|Urethral Stricture |
NCT03859024 |
| PD-1 Antibody Combined With COX Inhibitor in MSI-H/dMMR or High TMB Colorectal Cancer |
Recruiting |
Colorectal Cancer |
NCT03638297 |
| A Study of Lymphangiogenesis in Colorectal and Nasopharyngeal Cancer |
Unknown status |
Colorectal Cancer|Nasopharyngeal Cancer |
NCT00943891 |
| Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy |
Recruiting |
Prostate Cancer |
NCT00268476 |
| The Efficacy of TGF for Treating Osteoarthritis of the Knee |
Not yet recruiting |
Osteoarthritis, Knee |
NCT03562429 |
| Opioid-Free Shoulder Arthroplasty |
Completed |
Opioid Use|Shoulder Osteoarthritis|Avascular Necrosis|Rotator Cuff Tear|Arthritis |
NCT03540030 |
| Role of Prostaglandins in the Regulation of Brain Blood Flow |
Completed |
Healthy|Hypercapnia |
NCT00006318 |
| The Effect of Anti-inflammatory Analgesics on Pain Following Hallux Valgus Surgery |
Completed |
Postoperative Pain |
NCT00733421 |
| 12-Week No-Rofecoxib Plus Aspirin Endoscopy Study (0782-003) |
Terminated |
Arthritis, Rheumatoid|Osteoarthritis |
NCT00543465 |
| Dendritic Cells in Lung Cancer |
Completed |
Non Small Cell Lung Cancer |
NCT00442754 |
| Oral Tramadol Versus Oral Dexketoprofen for Reducing Pain During Office Hysteroscopy |
Not yet recruiting |
Hysteroscopy |
NCT03585036 |
| Evaluation of the Evolution of Imaging Markers of Cartilage Degradation in Patients With Knee Osteoarthritis Receiving DROGLICAN |
Unknown status |
Osteoarthritis, Knee |
NCT02821468 |
| Multimodal Analgesia With Acetaminophen vs. Narcotics Alone After Hip Arthroscopy |
Recruiting |
Hip Arthroscopy |
NCT03510910 |
| Comparison of Two Multimodal Analgesia Regimens in Total Knee Arthroplasty |
Terminated |
Postoperative Pain |
NCT03990038 |
| Pharmacogenetic and Pharmacokinetics of Naproxen and Associated Naproxen-esomeprazole |
Unknown status |
Poor Metabolizer Due to Cytochrome P450 CYP2C9 Variant|Poor Metabolizer Due to Cytochrome p450 CYP2C19 Variant |
NCT03092193 |
| Effects of Cox-II Inhibitor on Biochemical Markers in Cardiovascular-related Adverse Effects |
Unknown status |
Arthritis |
NCT00944866 |
| Optimizing Local Anesthetic Concentration for Continuous Femoral Nerve Blocks |
Completed |
Total Knee Arthroplasty|Knee Pain |
NCT00923598 |
| Use of Curcumin for Treatment of Intestinal Adenomas in Familial Adenomatous Polyposis (FAP) |
Completed |
Familial Adenomatous Polyposis |
NCT00927485 |
| MAST Trial: Multi-modal Analgesic Strategies in Trauma |
Active, not recruiting |
Nonspecific Pain Post Traumatic Injury |
NCT03472469 |
| Decreasing Narcotics in Advanced Pelvic Surgery |
Completed |
Narcotic Use|Pain|Constipation|Nausea |
NCT02110719 |
| Aspirin and Renal Disease Progression in Patients With Type 2 Diabetes |
Unknown status |
Diabetes |
NCT02895113 |
| Efficacy of Oral vs. Intravenous Acetaminophen |
Completed |
Postoperative Pain |
NCT02643394 |
| Association and Mechanism Between Cyclooxygenase-2 and Interleukin-6 in Gastric Cancer |
Unknown status |
Gastric Cancer |
NCT00172627 |
| Study Comparing 3 Different Treatments for Arthritis of the Lower Back (Lumbar Spinal Stenosis) |
Completed |
Lumbar Spinal Stenosis |
NCT01943435 |
| Optimization of Pre-operative Oral Analgesics in Patients Undergoing Ambulatory Minimally Invasive Hysterectomy |
Active, not recruiting |
Hysterectomy|Laparoscopy|Analgesia |
NCT03420794 |
| Nausea in Patients Receiving Hydromorphone vs Oxycodone After Total Hip Replacement Surgery |
Completed |
Nausea |
NCT02295124 |
| Protocol to Monitor the Neurological Development of Infants With Exposure in Utero From Birth to 15 Months in Tanezumab Clinical Studies |
Active, not recruiting |
Osteoarthritis|Cancer Pain|Recurrent Low Back Pain |
NCT03031938 |
| Long-term Observational Study of Subjects From Tanezumab Studies Who Undergo a Total Knee, Hip or Shoulder Replacement |
Completed |
Osteoarthritis |
NCT02674386 |
| Opiate Sparing Versus Opiate Based Following Shoulder Arthroplasty |
Enrolling by invitation |
Shoulder Surgery |
NCT04294680 |
| Protocol for the Treatment of Metastatic Ewing Sarcoma |
Active, not recruiting |
Ewing's Sarcoma (ES) |
NCT02727387 |
| A Study of the Kinetics of Lymphoid Cells in Patients With Monoclonal B-cell Lymphocytosis (MBL), Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL), Mantle Cell Lymphoma (MCL) and Healthy Volunteers |
Recruiting |
Lymphoma, Mantle-cell|CLL (Chronic Lymphocytic Leukemia)|Monoclonal B-Cell Lymphocytosis|Healthy Volunteers|Small Lymphocytic Lymphoma |
NCT01117142 |
| IPACK Nerve Block for Total Knee Arthroplasty |
Completed |
Arthropathy of Knee|Anesthesia |
NCT03921034 |
| Opioid-Sparing Protocol Comparing With Opioid-based Protocol After Bilateral Total Knee Arthroplasty |
Recruiting |
Knee Replacement|Postoperative Pain|Opioid Use |
NCT04314505 |
| Long-Term Analgesic Efficacy And Safety Of Tanezumab Alone Or In Combination With Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Versus NSAIDs Alone In Patients With Osteoarthritis Of The Knee Or Hip |
Terminated |
Osteoarthritis|Arthritis |
NCT00809354 |
| Evaluation of a Novel PET Radioligand to Image Cyclooxygenase-1 (COX-1) |
Recruiting |
Normal Physiology |
NCT03324646 |
| A2ALL-Patients Safety System Improvements in Living Donor Liver Transplantation |
Completed |
Liver Diseases|Hepatocellular Cancer|Endstage Liver Disease|Liver Cirrhosis |
NCT02073435 |
| First-line Treatment of Ewing Tumours With Primary Extrapulmonary Dissemination in Patients From 2 to 50 Years |
Recruiting |
Ewing Sarcoma Family of Tumors |
NCT03011528 |
| Minimal Opioid Use After Total Hip Replacement (THR) |
Recruiting |
Osteoarthritis, Hip |
NCT03090152 |
| Does Postoperative Gabapentin Reduce Pain, Opioid Consumption and Anxiety and Have a Positive Effect on Health Related Quality of Life After Radical Prostatectomy? |
Completed |
Prostate Cancer |
NCT00982800 |
| Total Hip Arthroplasty Outcomes With Regional and Multimodal Analgesia |
Completed |
Arthroplasty|Replacement, Hip|Arthritis |
NCT02186795 |
| Sutent and Radiation as Treatment for Limited Extent Metastatic Cancer |
Completed |
Cancer |
NCT00463060 |
| A Proof-of-concept Clinical Trial Assessing the Safety of the Coordinated Undermining of Survival Paths by 9 Repurposed Drugs Combined With Metronomic Temozolomide (CUSP9v3 Treatment Protocol) for Recurrent Glioblastoma |
Active, not recruiting |
Glioblastoma |
NCT02770378 |
| Crystalline Glucosamine Sulfate Therapy in Hand Osteoarthritis |
Completed |
Hand Osteoarthritis |
NCT03911570 |
| ERAS in Autologous Breast Reconstruction: A Pilot RCT |
Recruiting |
Breast Cancer |
NCT04306003 |
| Prospective Randomized Controlled Trial of Impact of Enhanced Recovery After Surgery(ERAS) for Outcomes of Total Knee and Hip Arthroplasty |
Recruiting |
Arthroplasty, Replacement, Knee, Hip |
NCT03869203 |
| Evaluation of Drug Utilization Patterns for Extended Release/Long Acting Opioids and Comparator Products |
Unknown status |
Opioid Related Disorders|Opiate Addiction|Narcotic Abuse|Drug Abuse |
NCT02925806 |
| Mechanisms of Human Cutaneous Microcirculation in Healthy Volunteers |
Completed |
Healthy Volunteers |
NCT00152724 |
| Effect of Combined Use of Naloxone and Tramacet on Postop Analgesia in Elderly Patients Having Joint Replacement Surgery |
Completed |
Hip Arthroplasty|Knee Arthroplasty|Spinal Anesthesia |
NCT00679614 |
| Molecular Phenotype Changes and Personalized Treatment for CRPC |
Unknown status |
Hormone Refractory Prostate Cancer |
NCT02208583 |
| Dexmedetomidine as Adjuvant for FNB in TKA |
Completed |
Nerve Block|Dexmedetomidine |
NCT03658421 |
| Predicting Patients' Response to Spinal Manipulation |
Completed |
Low Back Pain |
NCT00285649 |
| Anti-Tumor Necrosis Factor Therapy In Patients With Ankylosing Spondylitis |
Unknown status |
Ankylosing Spondylitis |
NCT02456363 |
| Individualizing Anti-Inflammatory Medications for Adults With Axial Spondyloarthritis: A Series of N-of 1 Trials |
Not yet recruiting |
Axial Spondyloarthritis |
NCT04115098 |
| YouScript IMPACT Registry |
Completed |
Adverse Drug Events|Adverse Drug Reactions|Drug Interaction Potentiation|Drug Metabolism, Poor, CYP2D6-RELATED|Drug Metabolism, Poor, CYP2C19-RELATED|Cytochrome P450 Enzyme Deficiency|Cytochrome P450 CYP2D6 Enzyme Deficiency|Cytochrome P450 CYP2C9 Enzyme Deficiency|Cytochrome P450 CYP2C19 Enzyme Deficiency|Cytochrome P450 CYP3A Enzyme Deficiency|Poor Metabolizer Due to Cytochrome P450 CYP2C9 Variant|Poor Metabolizer Due to Cytochrome P450 CYP2C19 Variant|Poor Metabolizer Due to Cytochrome P450 CYP2D6 Variant |
NCT02191358 |
| The Effect on Knee Joint Loads of Analgesic Use Compared With Exercise in Patients With Knee Osteoarthritis - An RCT |
Completed |
Osteoarthritis, Knee |
NCT01638962 |
| MT2013-31: Allo HCT for Metabolic Disorders and Severe Osteopetrosis |
Recruiting |
Mucopolysaccharidosis Disorders|Hurler Syndrome|Hunter Syndrome|Maroteaux Lamy Syndrome|Sly Syndrome|Alpha-Mannosidosis|Fucosidosis|Aspartylglucosaminuria|Glycoprotein Metabolic Disorders|Sphingolipidoses|Recessive Leukodystrophies|Globoid Cell Leukodystrophy|Metachromatic Leukodystrophy|Niemann-Pick B|Niemann-Pick C Subtype 2|Sphingomyelin Deficiency|Peroxisomal Disorders|Adrenoleukodystrophy With Cerebral Involvement|Zellweger Syndrome|Neonatal Adrenoleukodystrophy|Infantile Refsum Disease|Acyl-CoA Oxidase Deficiency|D-Bifunctional Enzyme Deficiency|Multifunctional Enzyme Deficiency|Alpha-methylacyl-CoA Racmase Deficiency|Mitochondrial Neurogastrointestingal Encephalopathy|Severe Osteopetrosis|Hereditary Leukoencephalopathy With Axonal Spheroids (HDLS |
NCT02171104 |
| A Study to Evaluate Safety and Efficacy of Elagolix in Participants With Endometriosis With Associated Moderate to Severe Pain |
Terminated |
Endometriosis |
NCT03343067 |
| Efficacy of Electro-acupuncture Versus Manual Acupuncture on Knee Osteoarthritis |
Unknown status |
Osteoarthritis Of Knee |
NCT03274713 |
| Pragmatic Clinical Trials in Scleroderma |
Not yet recruiting |
Scleroderma, Systemic|Sclerosis, Systemic |
NCT03610217 |
| Quality of Care for Knee and Hip Osteoarthritis in Elderly Patients |
Not yet recruiting |
Knee Osteoarthritis|Hip Osteoarthritis|Quality of Healthcare|Elderly |
NCT04170218 |
| High Dose Inorganic Selenium for Preventing Chemotherapy Induced Peripheral Neuropathy |
Not yet recruiting |
Chemotherapy-induced Peripheral Neuropathy|Recurrent Ovarian Carcinoma|Ovarian Cancer|Fallopian Tube Cancer|Primary Peritoneal Carcinoma |
NCT04201561 |
| Compare Outcomes Between Two Acellular Dermal Matrices |
Recruiting |
Breast Cancer |
NCT02891759 |
| A Randomized Trial of Behavioral Economic Approaches to Reduce Unnecessary Opioid Prescribing |
Completed |
Acute Pain |
NCT03825549 |