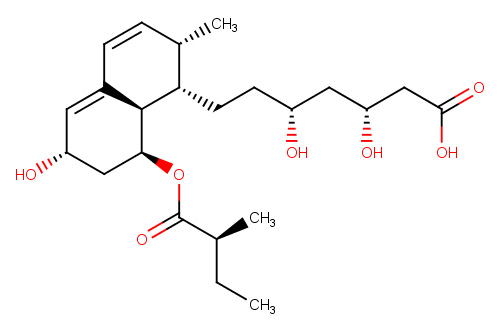
| Pravastatin to Prevent Preeclampsia |
Recruiting |
Preeclampsia|Obstetric Labor Complications|Hypertension in Pregnancy |
NCT03944512 |
| Indonesia Pravastatin to Prevent Preeclampsia Study |
Recruiting |
Pre-Eclampsia |
NCT03648970 |
| Pravastatin for Prevention of Preeclampsia |
Active, not recruiting |
Preeclampsia |
NCT01717586 |
| Effect of Pravastatin on Erythrocyte Membrane Fatty Acid Contents in Patients With Chronic Kidney Disease |
Completed |
Chronic Kidney Disease |
NCT02992548 |
| Effect of Pravastatin in the Subjects With Prediabetes or Early Diabetes |
Completed |
Diabetes Mellitus|Prediabetic State |
NCT02754739 |
| Drug-Drug Interaction Study With Pravastatin and Cyclosporine |
Completed |
Healthy |
NCT01497483 |
| Pravastatin Intervention to Delay Hepatocellular Carcinoma Recurrence |
Recruiting |
Hepatocellular Carcinoma|Liver Cirrhoses |
NCT03219372 |
| Effect of GDC-0810 on the Pharmacokinetics of Pravastatin in Healthy Female Subjects of Non-Childbearing Potential |
Completed |
Breast Cancer |
NCT02621957 |
| Effects of Pravastatin on Cholesterol, Inflammation and Cognition in Schizophrenia |
Completed |
Schizophrenia|Schizoaffective Disorders|Schizophreniform Disorders |
NCT01082588 |
| Single Dose Pravastatin Pharmacokinetics in Pediatric Peritoneal Dialysis Patients |
Terminated |
End Stage Renal Disease |
NCT00571194 |
| Pravastatin 80 mg Tablets Dosed in Healthy Subjects Under Non-Fasting Conditions |
Completed |
Healthy |
NCT00829309 |
| A Study of Zoledronic Acid, Pravastatin, and Lonafarnib for Patients With Progeria |
Completed |
Progeria|Hutchinson-Gilford Syndrome |
NCT00879034 |
| A Drug-Drug Interaction Study Between AZD1981 and Pravastatin to Study the Effect of AZD1981 on the Kinetics of Pravastatin |
Completed |
Drug Interaction |
NCT01254461 |
| Bioequivalence Study of Pravastatin Sodium Tablets 80 mg Under Fed Conditions |
Completed |
Healthy |
NCT01146093 |
| Study to Evaluate the Efficacy of Pravastatin on Survival and Recurrence of Advanced Gastroesophageal Cancer |
Unknown status |
Esophageal Cancer|Stomach Cancer |
NCT01038154 |
| Bioequivalence Study of Pravastatin Sodium Tablets 80 mg Under Fasting Conditions |
Completed |
Healthy |
NCT01146106 |
| Efficacy and Safety Study of Sorafenib Plus Pravastatin to Treat Advanced Hepatocarcinoma |
Completed |
Advanced Hepatocarcinoma |
NCT01418729 |
| A Study to Evaluate Daily Pravastatin, Fenofibrate or Pravafen in the Treatment of Combined Hyperlipidemia |
Completed |
Combined Hyperlipidemia |
NCT00459745 |
| The Effects of Pravastatin and Rosuvastatin on Coronary Plaques in Patients With Stable Angina Pectoris |
Unknown status |
Coronary Disease|Hypercholesterolemia |
NCT01325818 |
| The Influence of Raltegravir on Pravastatin Pharmacokinetics(GRAPPA) |
Completed |
HIV Infections |
NCT00665717 |
| Genetic Predictors of Variability in the Drug-drug Interaction Between Darunavir/Ritonavir and Pravastatin |
Completed |
HIV Infections|Hyperlipidemia |
NCT00630734 |
| Pravastatin Sodium 80 mg Tablets Under Fasting Conditions |
Completed |
Healthy |
NCT00830258 |
| Curative Efficacy of Pravastatine in Patients Presented Delayed Cutaneous and Subcutaneous Radio-induced Fibrosis |
Completed |
Fibrosis |
NCT01268202 |
| Treatment of the Hutchinson-Gilford Progeria Syndrome With a Combination of Pravastatin and Zoledronic Acid |
Completed |
Hutchinson-Gilford Progeria Syndrome |
NCT00731016 |
| Pravastatin and Alkali Therapy in Patients With Autosomal Dominant Polycystic Kidney Disease |
Recruiting |
Autosomal Dominant Polycystic Kidney Disease |
NCT04284657 |
| Statins: A New Therapeutic Option for Treatment of Patients With Endometriosis |
Withdrawn |
Endometriosis |
NCT02079974 |
| Pravastatin and Ventilatory Associated Pneumonia |
Completed |
Ventilator Associated Pneumonia |
NCT00702130 |
| Pravastatin or Atorvastatin Evaluation and Infection Therapy (TIMI22) |
Completed |
Actue Coronary Syndromes |
NCT00382460 |
| A Trial Comparing the Effect of Pravastatin and Rosuvastatin on Atherosclerosis Progression Measured by Carotid Intima Media Thickness in Patients With Coronary Artery Disease After Biolimus Eluting Stent (Nobori®) Implantation: CPR IMT |
Completed |
Coronary Artery Occlusive Disease |
NCT01872845 |
| Statin Adjunctive Therapy for TB (StAT- TB) |
Not yet recruiting |
Tuberculosis |
NCT03456102 |
| Idarubicin, Cytarabine, and Pravastatin Sodium in Treating Patients With Acute Myeloid Leukemia or Myelodysplastic Syndromes |
Completed |
Adult Acute Megakaryoblastic Leukemia (M7)|Adult Acute Minimally Differentiated Myeloid Leukemia (M0)|Adult Acute Monoblastic Leukemia (M5a)|Adult Acute Monocytic Leukemia (M5b)|Adult Acute Myeloblastic Leukemia With Maturation (M2)|Adult Acute Myeloblastic Leukemia Without Maturation (M1)|Adult Acute Myeloid Leukemia With 11q23 (MLL) Abnormalities|Adult Acute Myeloid Leukemia With Del(5q)|Adult Acute Myeloid Leukemia With Inv(16)(p13 |
NCT01831232 |
| Cyclosporine, Pravastatin Sodium, Etoposide, and Mitoxantrone Hydrochloride in Treating Patients With Relapsed or Refractory Acute Myeloid Leukemia |
Terminated |
Adult Acute Megakaryoblastic Leukemia (M7)|Adult Acute Minimally Differentiated Myeloid Leukemia (M0)|Adult Acute Monoblastic Leukemia (M5a)|Adult Acute Monocytic Leukemia (M5b)|Adult Acute Myeloblastic Leukemia With Maturation (M2)|Adult Acute Myeloblastic Leukemia Without Maturation (M1)|Adult Acute Myeloid Leukemia With 11q23 (MLL) Abnormalities|Adult Acute Myeloid Leukemia With Del(5q)|Adult Acute Myeloid Leukemia With Inv(16)(p13 |
NCT01342887 |
| Study of Zoledronic Acid, Pravastatin, and Lonafarnib for Patients With Progeria |
Enrolling by invitation |
Progeria |
NCT00916747 |
| Fed, Bioequivalence Study of Pravastatin Sodium 80 mg Tablets Versus Pravachol® 80 mg Tablets In Subjects |
Completed |
Therapeutic Equivalency |
NCT00648544 |
| A Study to Determine the Effect of WelChol Tablets on Cholesterol in Patients Who Have Been Taking Pravastatin for at Least 4 Weeks. |
Completed |
Hypercholesterolemia |
NCT00755352 |
| Fasting Bioequivalence Study of Pravastatin Sodium 80 mg Tablets Versus Pravachol® 80 mg Tablets In Normal Healthy Subjects |
Completed |
Therapeutic Equivalency |
NCT00650221 |
| A Study of Evacetrapib in Japanese and Non-Japanese Participants |
Completed |
Healthy Volunteers |
NCT01958489 |
| Drug-Drug Interaction Study of Vadadustat With Rosuvastatin, Sulfasalazine, Pravastatin, Atorvastatin and Simvastatin |
Completed |
Drug Interaction Potentiation |
NCT03801733 |
| A Study Comparing the Mechanisms of Action of Lifibrol and Pravastatin |
Completed |
Hypercholesterolemia|Hyperlipoproteinemia |
NCT01057654 |
| Pravastatin for Acute Myocardial Infarction With Minimally to Mildly Increased Levels of Serum Cholesterol Study |
Unknown status |
Acute Myocardial Infarction |
NCT00688922 |
| Pravastatin Sodium 40 mg Tablets Food Challenge Study |
Completed |
Healthy |
NCT00834847 |
| Clinical Trial to Evaluate the Efficacy and Safety of Pravafenix Cap to Verify the Superiority Than Atorvastatin |
Completed |
Combined Dyslipidemia |
NCT02166593 |
| Prevail-Us: A Study Of Pitavastatin 4 mg Vs. Pravastatin 40 mg In Patients With Primary Hyperlipidemia Or Mixed Dyslipidemia |
Completed |
Primary Dyslipidemia|Mixed Dyslipidemia |
NCT01256476 |
| A Study of the PK Interaction of CXA-10 With Pravastatin and Vytorin® in Healthy Males |
Completed |
Acute Kidney Injury |
NCT02547402 |
| Sorafenib Tosylate With or Without Pravastatin in Treating Patients With Liver Cancer and Cirrhosis |
Completed |
Liver Cancer |
NCT01075555 |
| StAT-TB (Statin Adjunctive Therapy for TB): A Phase 2b Dose-finding Study of Pravastatin in Adults With Tuberculosis |
Recruiting |
Tuberculosis|Pulmonary Tuberculosis |
NCT03882177 |
| Type II Diabetes Mellitus in Patients Exposed to Pravastatin and Paroxetine |
Completed |
Depression, Postpartum |
NCT01602913 |
| Statin Distribution |
Completed |
Drug Distribution |
NCT02360826 |
| Pravastatin Therapy in Patients With Active Crohn's Disease: A Pilot Study |
Unknown status |
Crohn's Disease |
NCT00599625 |
| Randomized Trial Sorafenib-Pravastatin Versus Sorafenib Alone for the Palliative Treatment of Child-Pugh A Hepatocellular Carcinoma |
Completed |
Child-Pugh A Hepatocellular Carcinoma |
NCT01903694 |
| Efficacy and Safety Study of JTT-705 in Combination With Pravastatin 40 mg in Patients With Type II Hyperlipidemia |
Completed |
Type II Hyperlipidemia |
NCT00688896 |
| Rosuvastatin Versus Pravastatin in HIV Patients Treated With Boosted Protease Inhibitors (PI) (ANRS126) |
Completed |
Hyperlipidemia|HIV Infections |
NCT00117494 |
| Pravastatin and Protease Inhibitors in HIV-Infected Patients |
Completed |
HIV Infection|Hypercholesterolemia |
NCT00221754 |
| Pravastatin for Hyperlipidaemia in HIV. |
Completed |
HIV Infections|Lipid Metabolism|Glucose Metabolism|Metabolic Abnormality|Lipodystrophy|Cardiovascular Disease |
NCT00227500 |
| Coadministration of Ezetimibe With Fenofibrate Versus Pravastatin Monotherapy for the Treatment of Hyperlipidaemia in HIV-infected Patients |
Unknown status |
HIV|Hyperlipidemia|HIV Infections |
NCT00843661 |
| Pravastatin Efficacy and Safety Trial in Hypercholesterolemic Patients With Chronic Liver Disease |
Completed |
Hypercholesterolemia |
NCT00529178 |
| Improving Symptoms of Schizophrenia and Schizoaffective Disorder by Supplementing Medications With Pravastatin |
Completed |
Schizophrenia|Schizoaffective Disorder |
NCT00177580 |
| Study to Compare the Efficacy and Safety of Pitavastatin and Pravastatin in Elderly Patients |
Completed |
Hypercholesterolemia or Combined Dyslipidemia |
NCT00257686 |
| A 12-Week Study Comparing Pitavastatin 4 mg vs. Pravastatin 40 mg in HIV-Infected Subjects |
Completed |
Dyslipidemia |
NCT01301066 |
| Bioavailability Study of Pravastatin Sodium 40 mg Tablets Under Fasting Conditions |
Completed |
Healthy |
NCT00834379 |
| Comparison of the Combination of Fenofibrate and Simvastatin Versus Pravastatin |
Completed |
Hyperlipidemia |
NCT00362206 |
| Additive Effects of Pravastatin and Valsartan |
Completed |
Hypertension|High Cholesterol |
NCT01004237 |
| Lipid Lowering Agents to Limit Lipid Oxidation and Activation of Clotting System in Nephrotic Syndrome |
Completed |
Nephrotic Syndrome|Hyperlipidemia |
NCT01845428 |
| S0919 Idarubicin, Cytarabine, and Pravastatin in Treating Patients With Relapsed Acute Myeloid Leukemia |
Active, not recruiting |
Leukemia |
NCT00840177 |
| Safety of Red Yeast Rice for High Cholesterol in Individuals With Statin Intolerance |
Completed |
Hypercholesterolemia|Statin-Associated Myopathy |
NCT00639223 |
| Safety and Effectiveness of Fenofibrate and Pravastatin in HIV-Positive Patients With Abnormal Blood Lipids |
Completed |
HIV Infections|Lipodystrophy |
NCT00006412 |
| Evaluation of the Efficacy and Safety of Rosuvastatin 5 mg Versus Pravastatin 40 mg and Atorvastatin 10 mg in Type IIa and IIb Hypercholesterolaemic Patients |
Completed |
Type IIa and IIb Hypercholesterolaemia |
NCT00631189 |
| Influence of Pravastatin on Carotid Artery Structure and Function in HIV-infected Patients Under Antiretroviral Therapy |
Completed |
HIV Infection|Carotid Atherosclerosis|Dyslipidemia |
NCT00147797 |
| Effects of Atorvastatin Versus Pravastatin on Platelet Inhibition by Clopidogrel |
Completed |
Ischemic Heart Disease|Acute Coronary Syndromes |
NCT00405717 |
| Pravastatin, Idarubicin, and Cytarabine in Treating Patients With Acute Myeloid Leukemia |
Completed |
Leukemia |
NCT00107523 |
| Monitoring and Treatment of Relapsed Leukemia Following Allogeneic Hematopoietic Stem Cell Transplantation in Children |
Active, not recruiting |
Leukemia |
NCT02484261 |
| STELLAR-Rosuvastatin vs. Atorvastatin, Pravastatin, Simvastatin Across Dose Ranges |
Completed |
Hypercholesterolemia |
NCT00654537 |
| Safety and Efficacy of Pravastatin in Relapsing-remitting Multiple Sclerosis |
Completed |
Relapsing-remitting Multiple Sclerosis |
NCT00200655 |
| Statin Therapy in Patients With Early Stage ADPKD |
Recruiting |
ADPKD|Autosomal Dominant Polycystic Kidney |
NCT03273413 |
| Study to Evaluate the Effect of SCY-078 (Ibrexafungerp) on the PK of Pravastatin in Healthy Subjects |
Not yet recruiting |
Pharmacokinetics |
NCT04092751 |
| Etoposide and Cisplatin or Carboplatin as First-Line Chemotherapy With or Without Pravastatin in Treating Patients With Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00433498 |
| Effect of Statins on Oxidative Stress and Endothelial Progenitor Cells |
Completed |
Diabetes Mellitus|Metabolic Syndrome X|Hypercholesterolemia |
NCT00166036 |
| Pharmacodynamics, Preliminary Pharmacokinetics and Tolerability of BIBB 515 BS or Pravastatin in Hyperlipemic Healthy Male Subjects |
Completed |
Healthy |
NCT02266485 |
| Yokohama Assessment of Fluvastatin, Pravastatin, Pitavastatin and Atorvastatin in Acute Coronary Syndrome (Yokohama-ACS) |
Completed |
Coronary Disease|Hypercholesterolemia |
NCT00549926 |
| Study to Investigate the Effect of BMS-986142 on the Pharmacokinetics (PK) of Methotrexate and Probe Substrate Cocktail in Healthy Patients |
Completed |
Rheumatoid Arthritis |
NCT02456844 |
| This Study Uses Ultrasound to Determine Whether Atorvastatin or Pravastatin Effects the Progression of Coronary Plaque. |
Completed |
Coronary Arteriosclerosis |
NCT00380939 |
| Effect of Pravastatin on Endothelial Dysfunction Following a Single High Fat Meal |
Unknown status |
Heart Diseases |
NCT00005117 |
| Cilostazol-Simvastatin Drug Interaction Study |
Unknown status |
Dyslipidemias|Peripheral Artery Disease |
NCT02431013 |
| COmparison and Modification in Neointimal Pattern Assessed by Optical Coherence Tomography With High Versus Moderate Efficacy Statin Treatment After Drug Eluting Stent Implantation: COMPASS Trial |
Completed |
Coronary Artery Disease |
NCT02155530 |
| The Statins on Glucose Homeostasis in Subjects With Impaired Fasting Glucose |
Recruiting |
Diabetes |
NCT01816997 |
| Palliative Treatment of Hepatocellular Carcinoma in Patient With CHILD B Cirrhosis |
Completed |
Hepatocellular Carcinoma|CHILD B |
NCT01357486 |
| Effect of Statin Therapy on Disease Progression in Autosomal Dominant Polycystic Kidney Disease (ADPKD) |
Completed |
Polycystic Kidney, Autosomal Dominant |
NCT00456365 |
| Japan Statin Treatment Against Recurrent Stroke (J-STARS) |
Completed |
Ischemic Stroke |
NCT00221104 |
| Efficacy and Safety of BMS-298585 Alone or in Combination With Pravastatin in Subjects With Mixed Dyslipidemia |
Completed |
Dyslipidemia |
NCT00245388 |
| Differential Metabolic Effects of Statins |
Completed |
Hypercholesterolemia |
NCT00546559 |
| Type III Dysbetalipoproteinemia |
Completed |
Hyperlipoproteinemia Type III |
NCT00214604 |
| Prevention of Renal and Vascular Endstage Disease Intervention Trial |
Completed |
Microalbuminuria|Cardiovascular Diseases|Renal Disease |
NCT03073018 |
| Aspirin Mono Therapy 12-months After Drug-eluting Stents Implantation |
Unknown status |
Drug-eluting Stent (DES) |
NCT01557075 |
| Effect of HAART Vs. Statin Treatment on Endothelial Function and Inflammation/Coagulation |
Withdrawn |
HIV-1 Infection |
NCT01515813 |
| Different Metabolic Effects of Statins |
Completed |
Hypercholesterolemia |
NCT01011127 |
| Effect of Statin Use on Aldosterone Secretion |
Recruiting |
Healthy Subjects |
NCT02871687 |
| Study to Determine the Potential DDIs When the Daclatasvir/Asunaprevir/BMS-791325 Three Drug Antiviral Combination Tablet (FDC) is Coadministered With a Cocktail of Cytochrome P450 (CYP) Probe Substrates and Transporter Probe Substrates (Digoxin and Pravastatin) in Healthy Subjects |
Completed |
Hepatitis C |
NCT02045966 |
| South Korean Pitavastatin Heart Failure Study |
Completed |
Chronic Heart Failure |
NCT00701285 |
| Anti-Oxidant Therapy In Chronic Renal Insufficiency (ATIC) Study |
Terminated |
Chronic Kidney Disease |
NCT00384618 |
| Effects of Concomitant Administration of BMS-986195 on Methotrexate, Caffeine, Montelukast, Flurbiprofen, Omeprazole, Midazolam, Digoxin, and Pravastatin |
Completed |
Rheumatoid Arthritis |
NCT03131973 |
| Do HMG CoA Reductase Inhibitors Affect Abeta Levels? |
Completed |
Alzheimer's Disease|Aging |
NCT00303277 |
| Combination Effects of High-dose Statin and Trimetazidine on Patients With Aspirin Mono Antiplatelet Therapy 12-months After Coronary Artery Bypass Surgery |
Completed |
Coronary Artery |
NCT01857921 |
| High Intensity Lipid Lowering Following Acute Coronary Syndromes for Persons Living With HIV |
Recruiting |
HIV Infection|Coronary Heart Disease |
NCT02841774 |
| A Study to Assess the Effect of RO4607381 in Patients With Relatively Low Levels of High Density Lipoprotein-Cholesterol (HDL-C) |
Completed |
Dyslipidemia |
NCT00697203 |
| Longitudinal Study of Multi-Analyte Profile for Dyslipidemia |
Unknown status |
Dyslipidemia |
NCT01441908 |
| Cardiovascular Prevention for Persons With HIV |
Completed |
HIV Infection|Cardiovascular Disease Risk |
NCT00982189 |
| Drug-Drug Interaction Study With PF-05089771 |
Completed |
Healthy |
NCT01934569 |
| Primary Prevention of Major Adverse Cardiac Events (MACE) With Standard and Intensive Statin Treatment in Patients With Diabetes: Survival and Cardiovascular Event Assessments |
Terminated |
Hypercholesterolemia|Type 2 Diabetes|Hypertension |
NCT01173939 |
| Comparison of Ezetimibe Added to Ongoing Statin Therapy Versus Doubling the Dose of Statin in the Treatment of Hypercholesterolemia (P04355) |
Completed |
Hypercholesterolemia |
NCT00652327 |
| Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese(MEGA Study) |
Completed |
Hyperlipidemia |
NCT00211705 |
| Statins and the Urinary Proteome |
Completed |
Statin Induced Proteinuria |
NCT00464503 |
| Carotid Intima-media Thickness in Japan Statin Treatment Against Recurrent Stroke(J-STARS Echo) |
Completed |
Ischemic Stroke |
NCT00361530 |
| Pharmacodynamics, Preliminary Pharmacokinetics and Tolerability of BIBB 1464 (Tablet) in Hyperlipemic Healthy Male Subjects |
Completed |
Healthy |
NCT02229773 |
| Pilot Study of a Multi-Drug Regimen for Severe Pulmonary Fibrosis in Hermansky-Pudlak Syndrome |
Terminated |
Hermansky-Pudlak Syndrome (HPS)|Pulmonary Fibrosis|Oculocutaneous Albinism|Platelet Storage Pool Deficiency|Metabolic Disease |
NCT00467831 |
| Prevention of Kidney Transplant Rejection |
Completed |
End-Stage Renal Disease|Chronic Allograft Nephropathy |
NCT00005010 |
| Cardiovascular Risk Markers and Response to Statins After Kawasaki Disease |
Withdrawn |
Kawasaki Disease |
NCT00305201 |
| Study on Effects of BMS-955176 on the Pharmacokinetics of Probe Substrates |
Completed |
HIV Infections |
NCT02578277 |
| Vytorin on Carotid Intima-media Thickness and Overall Rigidity |
Completed |
Cardiovascular Diseases |
NCT00738296 |
| Early Effects of Intensive Lipid Lowering Treatment With Ezetimibe/ Simvastatin (Vytorin®) Assessed by Virtual Histology-Intravascular Ultrasound (VH-IVUS) and Optical Coherence Tomography (OCT) on Plaque Characteristics in Patients With Acute Coronary Syndrome |
Completed |
Acute Coronary Syndrome |
NCT01857843 |
| AuTophagy Activation for Cardiomyopathy Due to Anthracycline tReatment (ATACAR)Trial |
Not yet recruiting |
Lymphoma |
NCT04190433 |
| Endothelial Dysfunction as a Risk Factor in HIV Study |
Completed |
HIV Infections |
NCT00039663 |
| Open Label Study for the Functional Characterization of Drug Metabolism and Transport |
Completed |
Genotype-related Drug Metabolism |
NCT01788254 |
| hsCRP in Japan Statin Treatment Against Recurrent Stroke (J-STARS hsCRP) |
Completed |
Ischemic Stroke |
NCT00361699 |
| Effects of Statin Medications on Mental Processes, Behavior, and Serotonin Levels |
Completed |
Dyslipidemias |
NCT00330980 |
| The Effect of Efavirenz and Nelfinavir on the Blood Levels of Certain Lipid-Lowering Drugs |
Completed |
HIV Infections|HIV Seronegativity|Lipodystrophy |
NCT00017758 |
| Quality of Life in Patients With Statin-Associated Myopathy |
Terminated |
Statin Adverse Reaction |
NCT00850460 |
| Serial EValuation of multiplE Coronary Artery Diseases by an Optical Coherence Tomography |
N/A |
N/A |
NCT01856374 |
| Left Ventricular Hypertrophy Reduction With Statins in Hypertensives Patients (MK0653A-168) |
Terminated |
Hypertension |
NCT00738972 |
| Evaluation of Myocardial Improvement in Patients Supported by Ventricular Assist Device Under Optimal Pharmacological Therapy |
Terminated |
Heart Failure |
NCT00402376 |
| Naturopathic Treatment for the Prevention of Cardiovascular Disease |
Completed |
Cardiovascular Disease |
NCT00718796 |
| Efficacy and Safety of Prescription Omega-3 Fatty Acid Added to Stable Statin Therapy in Patients With Type 2 Diabetes and Hypertriglyceridemia |
Completed |
Diabetes Mellitus, Type 2|Hypertriglycemia |
NCT02305355 |
| Statin Recapture Therapy Before Coronary Artery Bypass Grafting |
Completed |
Coronary Artery Disease |
NCT01715714 |
| Aggressive Treatment of Metabolic Syndrome in Patients Receiving Clozapine for Schizophrenia |
Terminated |
Metabolic Syndrome |
NCT00794963 |
| Observational Study of Approaches to Lipid-Lowering Therapy in Russian Patients With Coronary Heart Disease <<Treat to Goal>> (Study P05464) |
Completed |
Coronary Heart Disease|Hypercholesterolemia |
NCT00730132 |
| A Study on Possible Interactions Between Protease Inhibitors (Anti-HIV Drugs) and Drugs Which Lower the Level of Fat in Your Blood |
Completed |
HIV Infections |
NCT00000941 |
| Repeated Dose Study for the Investigation of Heritability of and Genetic Influences on Drug Pharmacokinetics |
Completed |
Drug Biotransformation|Membrane Transport |
NCT01845194 |
| Statin Interchange by Pharmacist Collaborative Practice Agreement (CPA) |
Completed |
Hypercholesterolemia |
NCT01222182 |
| Neointimal Coverage After Implantation of Biolimus Eluting Stent With Biodegradable Polymer: Optical Coherence Tomographic Assessment According to the Treatment of Dyslipidemia and Hypertension and the Types of Implanted Drug-eluting Stents |
Completed |
Stable Angina or Acute Coronary Syndrome Considered for Percutaneous Coronary Intervention With Dyslipidemia or Hypertension |
NCT01502904 |
| High Potency Statins and Acute Kidney Injury |
Completed |
Hypercholesterolemia |
NCT02518516 |
| High Potency Statins and the Risk of Diabetes |
Completed |
Diabetes Mellitus, Type 2|Cardiovascular Disease |
NCT02518503 |
| Pitavastatin on Carotid Intima-media Thickness |
Unknown status |
Hyperlipidemia|Carotid Artery Diseases |
NCT00711919 |
| Fluvastatin in Adults With Dislipidemia With History of Muscle Problems |
Completed |
Dyslipidemia |
NCT00125125 |
| Efficacy and Safety of Colesevelam in Pediatric Patients With Genetic High Cholesterol |
Completed |
Hypercholesterolemia |
NCT00145574 |
| Prevention of Atherosclerosis and Heart Disease in Patients With Systemic Lupus Erythematosis (SLE) |
Completed |
Systemic Lupus Erythematosus|Lupus |
NCT00054938 |
| High-Density Lipoprotein (HDL) Cholesterol Increased Plaque Stabilization in the Elderly |
Completed |
Atherosclerosis|Cardiovascular Disease |
NCT00127218 |
| To Explore the Treatment Effect of Various Commercially Available Statins on Patients With Hyperlipidemia |
Completed |
Hyperlipidemias |
NCT00726362 |
| Evaluation of the Efficacy of Rosuvastatin in Daily Practice (TARGET) |
Completed |
Hypercholesterolemia|Coronary Heart Disease |
NCT00396110 |
| The Triglyceride Lowering Effect of an Omega-3 Fat (DHA) in Addition to Statin Therapy for Patients With CAD or Diabetes |
Completed |
Hypertriglyceridemia (TG>200<500)|Hyperlipidemia|Coronary Artery Disease|Coronary Risk Equivalent|Diabetes |
NCT00360217 |
| Harvard Atherosclerosis Reversibility Project (HARP) |
Completed |
Cardiovascular Diseases|Coronary Disease|Heart Diseases|Myocardial Ischemia |
NCT00000461 |
| Ekvasis of Atorvastatin (Antorcin®) Treatment in Patients With Acute Cardiovascular Events |
Completed |
Cardiovascular Disorders |
NCT01770210 |
| Effect of Pitavastatin on Erythrocyte Membrane Fatty Acid Contents in Patients With Chronic Kidney Disease |
Unknown status |
Chronic Kidney Disease |
NCT02863185 |
| Efficacy and Safety of PRC-4016 in Subjects With Mixed Dyslipidemia |
Terminated |
Dyslipidemia |
NCT01972178 |
| Erythrocyte-bound Apolipoprotein B After Withdrawal of Statin Therapy |
Completed |
Hyperlipidemia|Atherosclerosis |
NCT01634906 |
| Effect of Succinylcholine on Patients Using Statins |
Completed |
Pain |
NCT00986583 |
| Study to Investigate the Efficacy and Safety of Cannabis Oil for the Treatment of Subjects With Hidradenitis Suppurativa |
Not yet recruiting |
Hidradenitis Suppurativa |
NCT03929835 |
| Evaluation of Patients With Statin Myopathy Using an N of 1 Trial Design |
Completed |
Statin Myopathy |
NCT01259791 |
| A RAndomizeD Intervention for Cardiovascular and Lifestyle Risk Factors in Prostate Cancer Patients |
Recruiting |
Prostate Cancer|Cardiovascular Disease |
NCT03127631 |
| Clinical Study to Evaluate Adherence Improvement Fixed-dose Combination of Olostar Tab. in Patients With Hypertension and Dyslipidemia |
Completed |
Hypertension|Dyslipidemias |
NCT04061824 |
| Role of Hydroxychloroquine to Improve Endothelial Dysfunction in Patients With Rheumatoid Arthritis |
Completed |
Rheumatoid Arthritis |
NCT03085940 |
| Efficacy and Safety Evaluation of Alirocumab in Patients With Heterozygous Familial Hypercholesterolemia or High Cardiovascular Risk Patients With Hypercholesterolemia on Lipid Modifying Therapy (ODYSSEY JAPAN) |
Completed |
Hypercholesterolemia |
NCT02107898 |
| Arterial Disease Multifactorial Intervention Trial (ADMIT) |
Completed |
Arterial Occlusive Diseases|Cardiovascular Diseases|Heart Diseases|Vascular Diseases|Diabetes Mellitus |
NCT00000539 |
| Evaluation of ETC-1002 vs Placebo in Patients Receiving Ongoing Statin Therapy |
Completed |
Hypercholesterolemia |
NCT02072161 |
| Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) |
Completed |
Cardiovascular Diseases|Coronary Disease|Diabetes Mellitus|Heart Diseases|Hypercholesterolemia|Hypertension|Myocardial Infarction|Myocardial Ischemia|Heart Failure |
NCT00000542 |
| An Efficacy and Safety Study of Alirocumab in Children and Adolescents With Heterozygous Familial Hypercholesterolemia |
Recruiting |
Hypercholesterolaemia |
NCT03510884 |
| An Efficacy and Safety Study of Alirocumab in Children and Adolescents With Homozygous Familial Hypercholesterolemia |
Completed |
Hypercholesterolemia |
NCT03510715 |
| A 12 Week Study of MK0653A in Patients Who Have Been Hospitalized for a Possible Heart Problem (0653A-808) |
Completed |
Hypercholesterolemia |
NCT00132717 |
| Intensive Statin Treatment in Chinese Coronary Artery Disease Patients Undergoing PCI |
Completed |
Non-ST Segment Elevation Myocardial Infarction|Unstable Angina Pectoris|Stable Angina Pectoris |
NCT01293097 |
| Efficacy of Lapaquistat Acetate Co-Administered With Statins in Subjects With Hypercholesterolemia |
Terminated |
Hypercholesterolemia |
NCT00532311 |
| A Study of LY3015014 in Participants With High Cholesterol |
Completed |
Hypercholesterolemia |
NCT01890967 |
| Optimal Blood Pressure and Cholesterol Targets for Preventing Recurrent Stroke in Hypertensives |
Recruiting |
Stroke|Transient Ischemic Attack|Hypertension |
NCT01563731 |
| A Study of AMR101 to Evaluate Its Ability to Reduce Cardiovascular Events in High Risk Patients With Hypertriglyceridemia and on Statin. The Primary Objective is to Evaluate the Effect of 4 g/Day AMR101 for Preventing the Occurrence of a First Major Cardiovascular Event. |
Completed |
Cardiovascular Diseases |
NCT01492361 |
| Efficacy and Safety of AMR101 (Ethyl Icosapentate) in Patients With Fasting Triglyceride (Tg) Levels ≥ 500 and ≤ 2000 mg/dL |
Completed |
Hypertriglyceridemia |
NCT01047683 |
| Effect of AMR101 (Ethyl Icosapentate) on Triglyceride (Tg) Levels in Patients on Statins With High Tg Levels (≥ 200 and < 500 mg/dL) |
Completed |
Hypertriglyceridemia |
NCT01047501 |
| Studies of Traditional Chinese Medicine Clinical Efficacy Evaluation Index |
Completed |
Coronary Heart Disease|Stable Angina Pectoris |
NCT01502943 |
| Belatacept Evaluation of Nephroprotection and Efficacy as First-line Immunosuppression (BENEFIT) |
Completed |
Kidney Transplantation|Chronic Kidney Failure |
NCT00256750 |