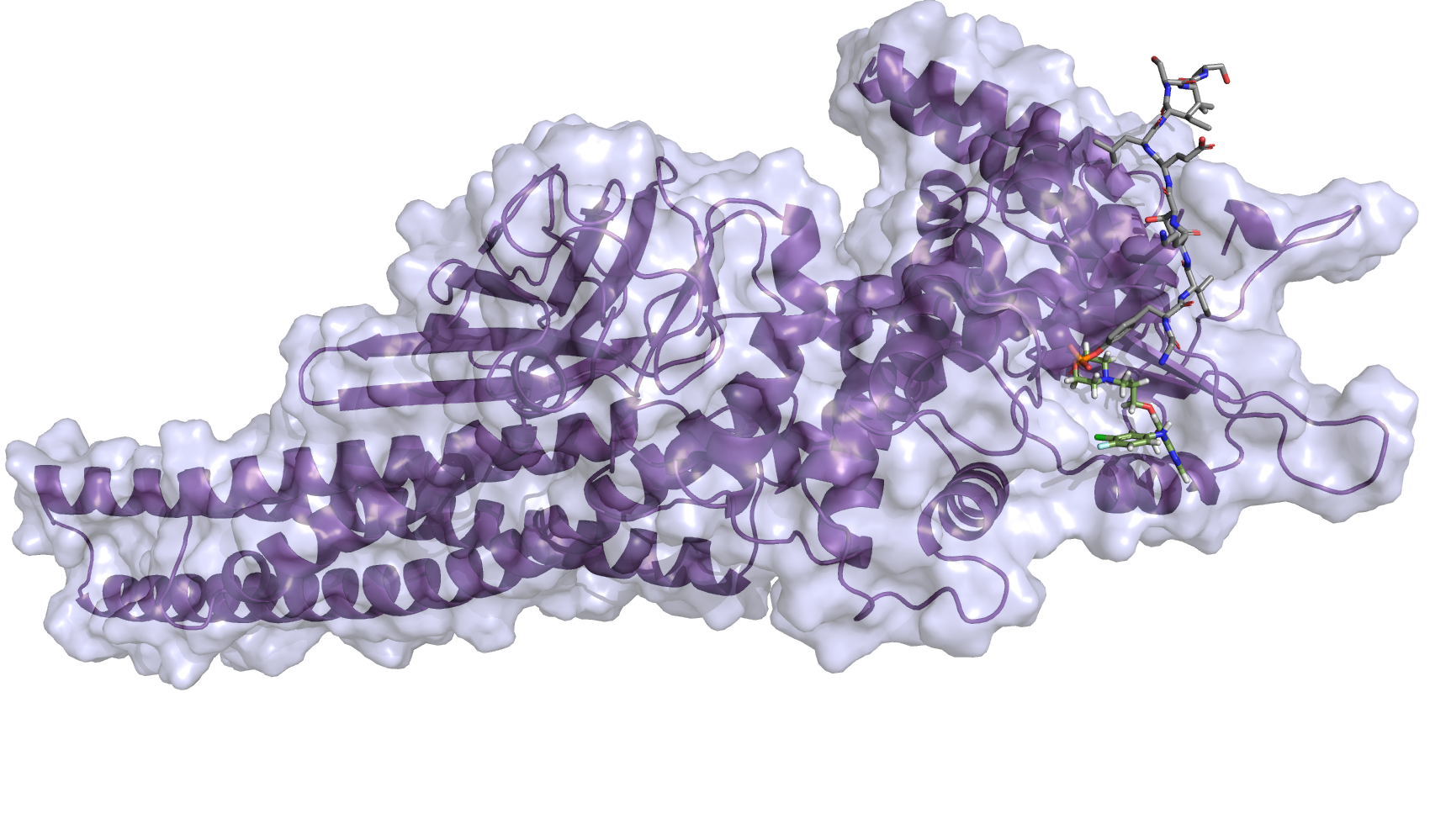
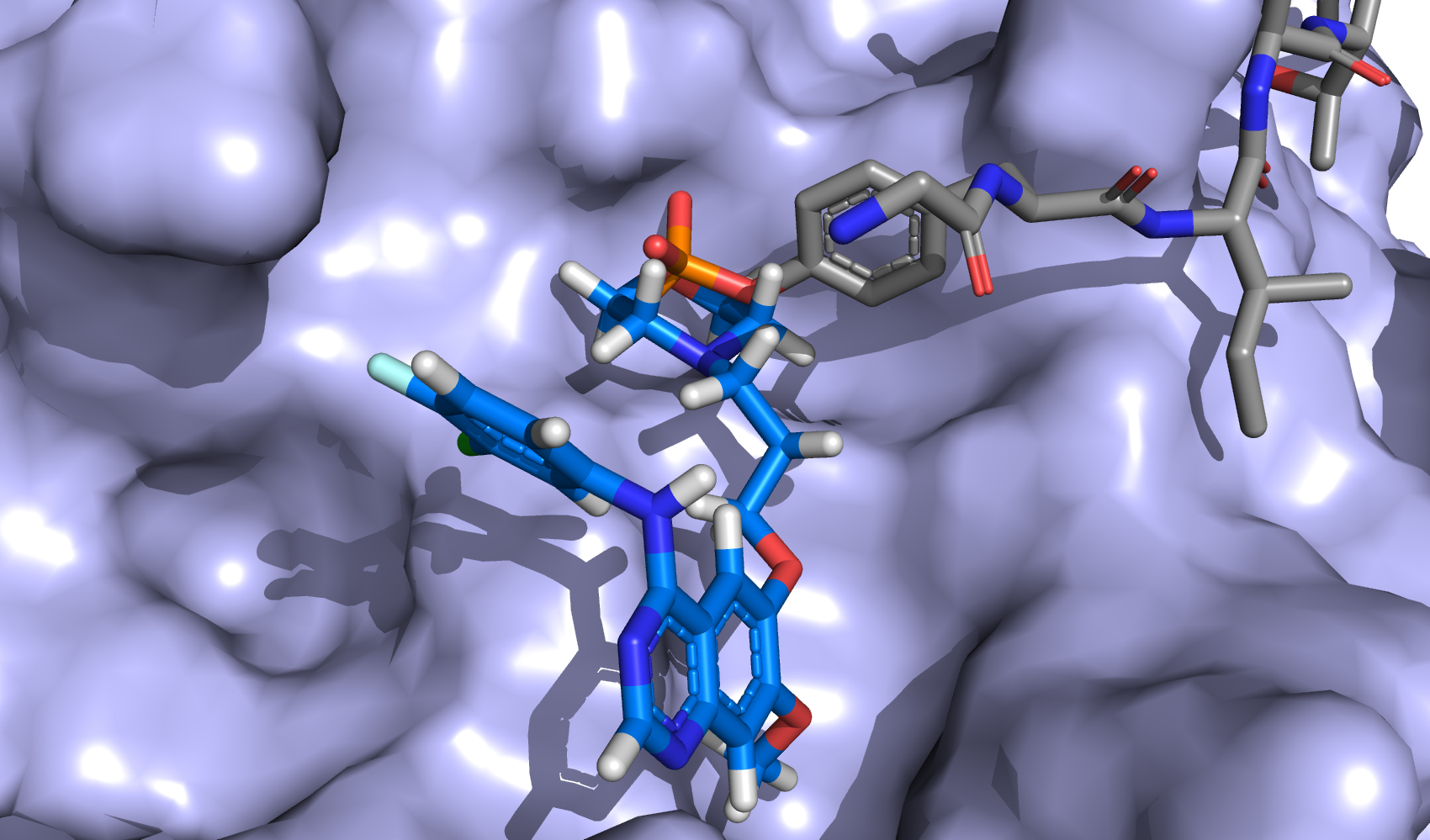
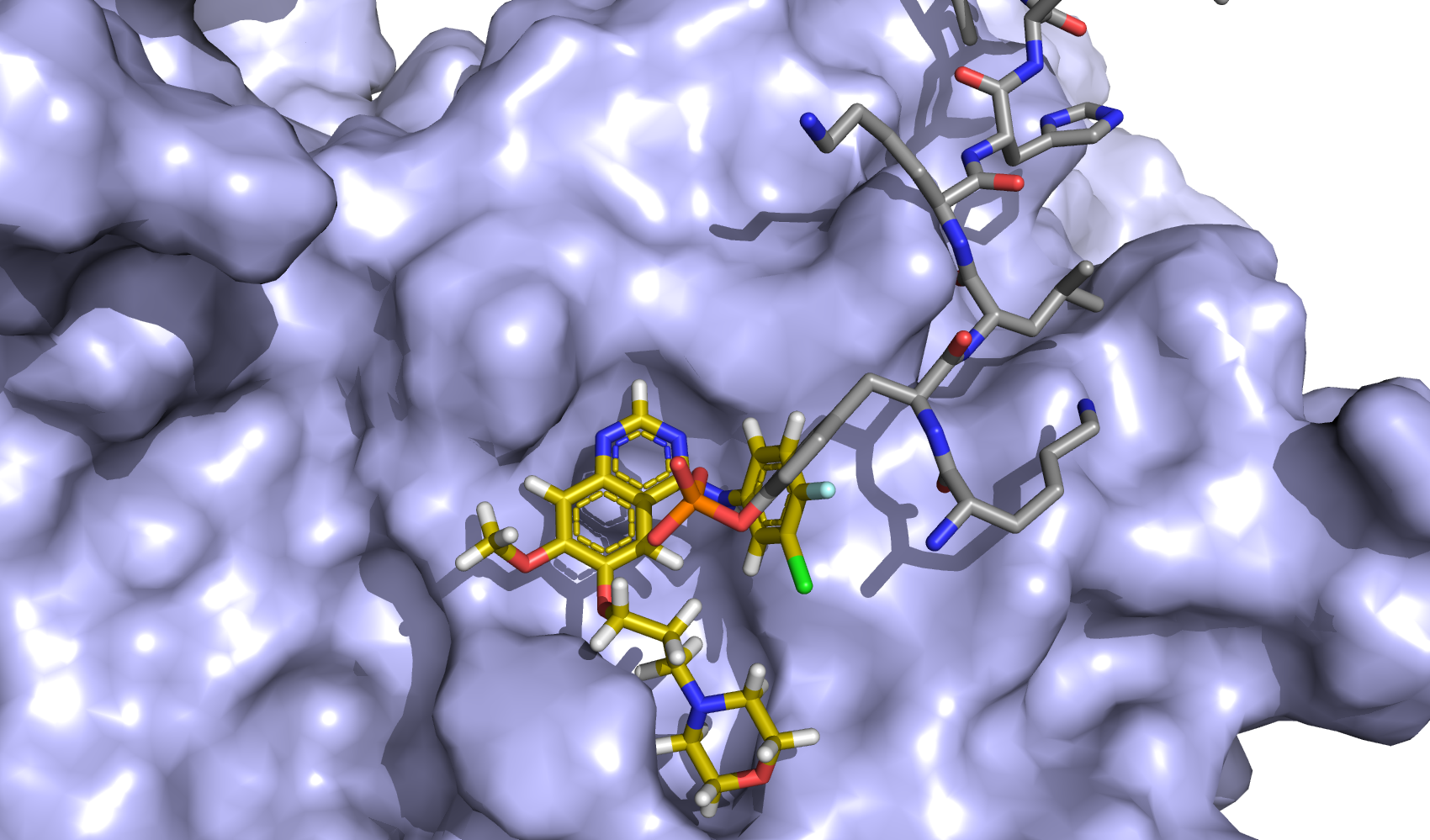
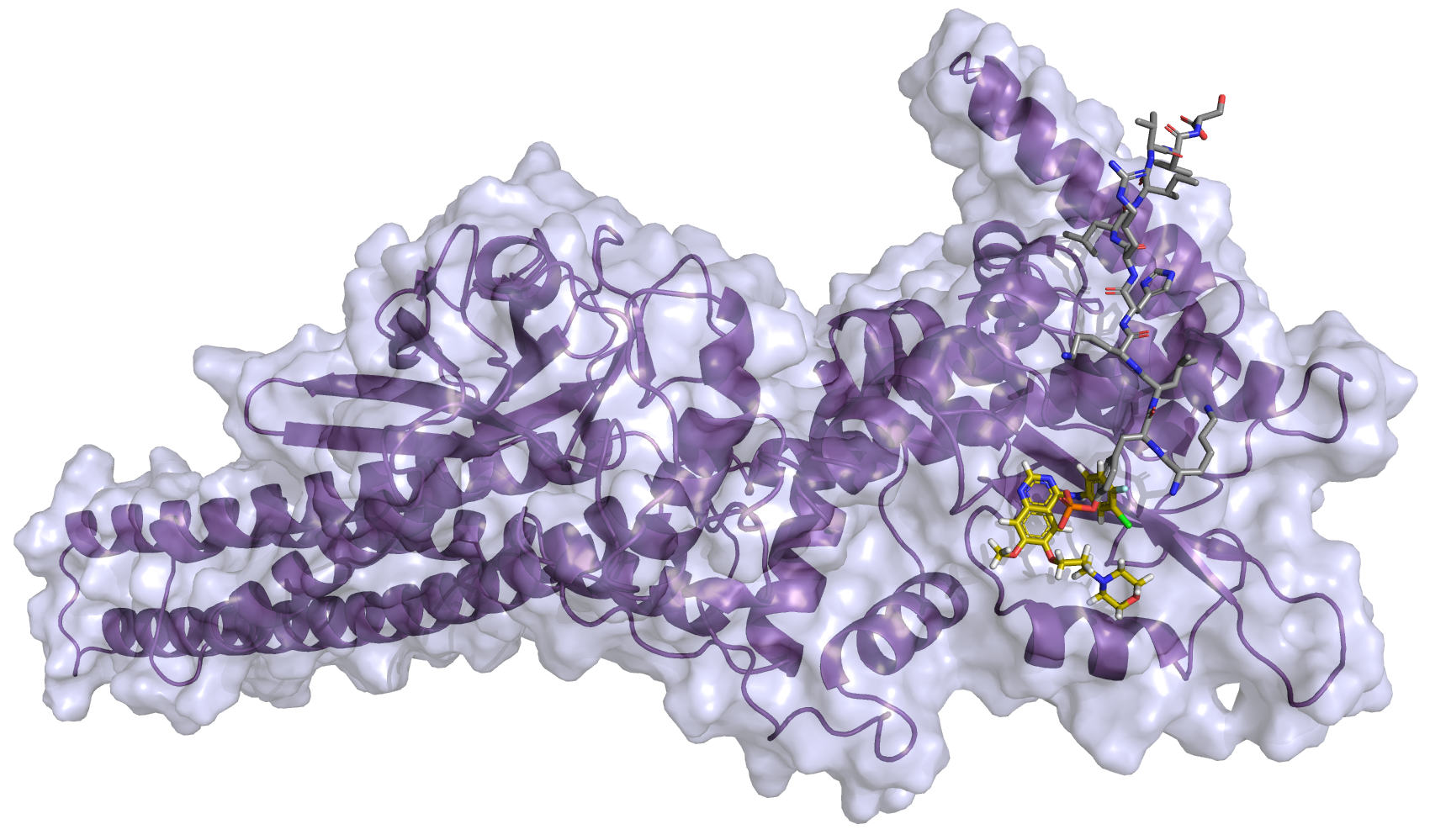
| S-1 Plus Gefitinib Versus Gefitinib Monotherapy in Patients With EGFR-sensitive Mutation Advanced Non-squamous NSCLC |
Not yet recruiting |
EGFR-sensitive Mutation-positive Advanced Non-squamous Non-small Cell Lung Cancer Treated With S-1plus Gefitinib Versus Gefitinib Monotherapy |
NCT03457337 |
| PRe-Operative Gefitinib in Resectable EGFR Mutation Positive Lung Cancer With Sector Sequencing for Biomarker Discovery |
Recruiting |
Non-small Cell Lung Cancer |
NCT02804776 |
| Bone Metastasis on the Survival of Gefitinib Effective Patients |
Completed |
Overal Survival, Non-small Cell Lung Cancer |
NCT03157310 |
| Osimertinib and Gefitinib in EGFR Inhibitor naĂŻve Advanced EGFR Mutant Lung Cancer |
Recruiting |
Non-Small Cell Lung Cancer |
NCT03122717 |
| First Line Gefitinib by FDG-PET Metabolic Response |
Unknown status |
Non-small Cell Lung Cancer |
NCT01510990 |
| Gefitinib and Berberine in the First-line Treatment of Lung Adenocarcinoma With EGFR Mutation |
Unknown status |
Lung Adenocarcinoma|EGFR Mutation |
NCT03486496 |
| Gefitinib Combine Radiotherapy as Therapy for Patients With NSCLC Harbouring Sensitive Mutations of EGFR |
Not yet recruiting |
Lung Cancer |
NCT03381430 |
| ASK120067 Versus Gefitinib as First-line Treatment for EGFRm Locally Advanced or Metastatic NSCLC |
Recruiting |
Locally Advanced or Metastatic NSCLC |
NCT04143607 |
| Blood Detection of EGFR Mutation For Iressa Treatment |
Unknown status |
Lung Adenocarcinoma |
NCT02282267 |
| A Study of EGF816 and Gefitinib in TKI-naĂŻve EGFR-mutant Non-Small Cell Lung Cancer |
Recruiting |
Lung Cancer |
NCT03292133 |
| A Study of Gefitinib With or Without Apatinib in Patients With Advanced Non-squamous Non-Small-Cell Lung Cancer Harboring EGFR Mutations |
Recruiting |
EGFR Tyrosine Kinase Inhibitors Plus VEGFR Inhibitors |
NCT02824458 |
| Preoperative Gefitinib for EGFR Mutant II-IIIa NSCLC |
Unknown status |
Non-small-cell Lung Cancer |
NCT01833572 |
| Study of Gefitinib Retreatment in Non-Small Cell Lung Cancer (NSCLC) |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00824746 |
| High-Dose,Pulsatile Erlotinib/Gefitinib for Advanced NSCLC Patients After Failure of Standard Dose EGFR-TKIs |
Unknown status |
Self Efficacy|Drug Toxicity |
NCT01965275 |
| Hydroxychloroquine and Gefitinib to Treat Lung Cancer |
Unknown status |
Non-small Cell Lung Cancer |
NCT00809237 |
| Gefitinib in Combination With Anlotinib or Placebo in Previously Untreated EGFR-mutant NSCLC |
Recruiting |
Lung Cancer, Nonsmall Cell |
NCT04028778 |
| Third-line Treatment of Gefitinib in NSCLC Patients |
Completed |
Non Small Cell Lung Cancer |
NCT01933347 |
| Iressa Re-Challenge in Advanced NSCLC EGFR M+ Patients Who Responded to Gefitinib USed as 1st Line or Previous Treatment |
Completed |
Lung Cancer |
NCT01530334 |
| Ningetinib (CT053PTSA) Plus Gefitinib in Stage IIIB or IV NSCLC Patients With EGFR Mutation and T790M Negative |
Recruiting |
Non-small Cell Lung Cancer |
NCT03758287 |
| A Phase II Study of Fruquintinib in Combination With Gefitinib in Patients With Advanced Non-small Cell Lung Cancer |
Completed |
NSCLC |
NCT02976116 |
| Bioequivalence Study of Gefitinib Tablets Under Fed Conditions |
Completed |
Healthy |
NCT03050177 |
| The Continuation of Gefitinib Treatment Beyond Progression in Non-small Cell Lung Cancer Patients With EGFR Mutation |
Unknown status |
Non-small Cell Lung Cancer |
NCT03399669 |
| Bioequivalence Study of Gefitinib Tablets Under Fasting Conditions |
Completed |
Bioequivalence Study |
NCT03050164 |
| NEoadjuvant Gefitinib fOllowed by Surgery and gefiTinib In unresectAble sTage III NSCLC With EGFR Mutations. |
Recruiting |
Lung Cancer |
NCT02347839 |
| Epidermal Growth Factor Receptor (EGFR) Status Based Gefitinib Neoadjuvant Therapy in Non Small Cell Lung Cancer (NSCLC) |
Suspended |
Non Small Cell Lung Cancer |
NCT00986284 |
| Gefitinib Versus Vinorelbine/Platinum as Adjuvant Treatment in Stage II-IIIA(N1-N2) NSCLC With EGFR Mutation |
Active, not recruiting |
Non-small Cell Lung Cancer |
NCT01405079 |
| Chemotherapy Plus Gefitinib for Advanced Lung Adenocarcinoma and Sensitive EGFR Mutations: a Randomized Controlled Trial |
Unknown status |
Lung Adenocarcinoma |
NCT02951637 |
| Efficacy and Safety Study of Gefitinib in Squamous NSCLC Patients Who Failed First-Line Chemotherapy |
Unknown status |
Squamous Cell Carcinoma of Bronchus |
NCT01485809 |
| High Dose Gefitinib for the Treatment of Carcinomatous Meningitis in Adult Patients With Non-Small Cell Lung Cancer and Known or Suspected EGFR Mutations |
Completed |
Non-Small Cell Lung Cancer |
NCT00372515 |
| Phase II Study of Gefitinib Plus Nimotuzumab Versus Gefitinib in Non-small Cell Lung Cancer |
Completed |
Non Small Cell Lung Cancer (NSCLC) |
NCT01498562 |
| Phase I of Vorinostat-Iressa Combined Therapy on Resistance by BIM Polymorphysim in EGFR Mutant Lung Cancer |
Unknown status |
Non-Small-Cell Lung Carcinoma |
NCT02151721 |
| Tolerability and Efficacy of Tremelimumab in Combination With Gefitinib in NSCLC Patients |
Completed |
Non Small Cell Lung Cancer |
NCT02040064 |
| DS-1205c With Gefitinib for Metastatic or Unresectable Epidermal Growth Factor Receptor (EGFR)-Mutant Non-Small Cell Lung Cancer |
Active, not recruiting |
Non Small Cell Lung Cancer |
NCT03599518 |
| Gefitinib or Docetaxel as Second Line Therapy for Wild-type Epidermal Growth Factor Receptor (EGFR) NSCLC |
Unknown status |
Non-small Cell Lung Cancer |
NCT01755923 |
| Study of Iressa in Patients With Relapsed or Refractory Acute Myelogenous Leukemia |
Completed |
Myelogenous Leukemia, Acute |
NCT00130702 |
| Pemetrexed Combined With Synchronous Gefitinib as Adjuvent Therapy in Patient With EGFR Mutant Lung Adenocarcinoma |
Unknown status |
Lung Neoplasms |
NCT02518802 |
| Phase I Study of the Combination of Anlotinib With Gefitinib |
Not yet recruiting |
Non-squamous Non-small Cell Lung Cancer |
NCT03602027 |
| The Safety and Effects of Gefitinib in Triple-negative,EGFR Positive Metastatic Breast Cancer |
Unknown status |
Breast Cancer |
NCT01732276 |
| Assess the Efficacy, Safety and Tolerability of Gefitinib (Iressa® 250mg) as Maintenance Therapy in Locally Advanced or Metastatic (Stage IIIB/IV) Non Small Cell Lung Cancer (NSCLC) |
Completed |
Non-small Cell Lung Cancer (NSCLC) |
NCT00770588 |
| Phase II Study of Skin Toxicity Dosing of IRESSA (Gefitinib) in Squamous Cell Carcinoma of the Head and Neck |
Completed |
Head and Neck Neoplasms |
NCT00519077 |
| A Trial of Gefitinib in Combination With BKM120 in Patients With Advanced Non-Small Cell Lung Cancer, With Enrichment for Patients Whose Tumours Harbour Molecular Alterations of PI3K Pathway and Known to Overexpress EGFR |
Active, not recruiting |
Non-Small Cell Lung Cancer|Solid Tumors |
NCT01570296 |
| CIK Cell Transfusion Plus Gefitinib As Second Or Third-Line Treatment for Advanced Adenocarcinoma Non-Small Cell Lung Cancer |
Terminated |
Non-Small Cell Lung Cancer |
NCT01871480 |
| A Study Of Nasopharyngeal Carcinoma (NPC) Treated With Celecoxib And ZD1839 |
Completed |
Nasopharyngeal Carcinoma |
NCT00212108 |
| Vinorelbine-ifosfamide Versus Gefitinib for EGFR Gene Mutation Negative Non-small Cell Lung Cancer Patients |
Unknown status |
Non-small Cell Lung Cancer|Effects of Chemotherapy |
NCT01749072 |
| Efficacy, Safety, Tolerability of Gefitinib as 1st Line in Caucasian Patients With EGFR Mutation Positive Advanced NSCLC |
Completed |
Caucasian Patients With EGFR Mutation Positive Advanced NSCLC |
NCT01203917 |
| Gefitinib Combined With Radiotherapy in Elderly Patients With Esophageal Cancer |
Unknown status |
Esophageal Cancer |
NCT01291823 |
| Efficacy of Gefitinib for Brain Metastasis of Non-Small Cell Lung Cancer |
Unknown status |
Non-Small Cell Lung Cancer|Brain Metastasis |
NCT00614809 |
| the Clinical Trial of Gefitinib(Non - Small Cell Lung Cancer) |
Not yet recruiting |
Non-small Cell Lung Cancer |
NCT03264794 |
| Gefitinib With or Without Pemetrexed/Cisplatin in Brain Metastases From Non-small Cell Lung Cancer |
Unknown status |
Non-small Cell Lung Cancer|Brain Metastases|EGFR Mutation |
NCT01951469 |
| Study With Gefitinib in Combination With Olaparib (AZD2281) Versus Gefitinib Alone |
Completed |
Non Small Cell Lung Cancer |
NCT01513174 |
| Radiotherapy Combined With Iressa for EGFR Mutation Positive Patients With Locally Advanced Non-small Cell Lung Cancer (NSCLC) |
Unknown status |
Non-small Cell Lung Cancer |
NCT01391260 |
| GEfitinib Plus viNOrelbine in Advanced EGFR Mutated NSCLC. GENOA Trial |
Unknown status |
Non Small Cell Lung Cancer |
NCT02319577 |
| ARCHER1050: A Study of Dacomitinib vs. Gefitinib in 1st-Line Treatment Of Advanced NSCLC. |
Active, not recruiting |
Non-small Cell Lung Cancer With EGFR-Activating Mutations |
NCT01774721 |
| Gefitinib 500mg Versus 250mg in Patients With NSCLC With Stable Disease After a Month Treatment of Gefitinib 250mg |
Completed |
Non-small Cell Lung Cancer |
NCT01017679 |
| Postoperative Radiotherapy Plus Iressa or Radiotherapy Plus Cisplatin and Iressa for Advanced Head & Neck Cancer |
Completed |
Head and Neck Cancer |
NCT00681967 |
| Dose Defining Study For MK-2206 Combined With Gefitinib In Non Small Cell Lung Cancer (NSCLC) |
Unknown status |
Non Small Cell Lung Cancer |
NCT01147211 |
| Real World Study on Erlotinib/Gefitinib Combined With Bevacizumab in Advanced Non-aquamous Non-small Cell Lung Cancer |
Recruiting |
Non Small Cell Lung Cancer |
NCT03647592 |
| Phase III Trial to Evaluate the Elortinib vs Gefitinib in Advanced NSCLC With EGFR Exon 19 or 21 Mutations |
Completed |
Thoracic Neoplasms |
NCT01024413 |
| Gefitinib Combined With Chemotherapy or Antiangiogensis in Patients With Bim Deletion or Low EGFR Mutation Abundance |
Unknown status |
Non-small-cell Lung Cancer |
NCT02930954 |
| Gefitinib With Chemotherapy or Anti-angiogenesis in NSCLC Patients With Bim Deletion or Low EGFR Mutation Abundance |
Unknown status |
Non-small-cell Lung Cancer |
NCT03267654 |
| Intercalating and Maintenance Use of Iressa Versus Chemotherapy in Selected Advanced Non Small Cell Lung Cancer |
Completed |
Non-small Cell Lung Cancer |
NCT01404260 |
| Gefitinib and Etoposide in Treating Patients With Advanced Prostate Cancer That Did Not Respond to Hormone Therapy |
Terminated |
Prostate Cancer |
NCT00483561 |
| Iressa Study in Patients With Salivary Gland Cancer |
Completed |
Salivary Gland Cancer |
NCT00509002 |
| Gefitinib Versus Pemetrexed for Previously Treated NSCLC Patients |
Completed |
Previous Treated Metastatic Non-small Cell Lung Cancer |
NCT01783834 |
| A Study of Pemetrexed and Gefitinib Versus Gefitinib in Non-Small Cell Lung Cancer (NSCLC) |
Completed |
Carcinoma, Non Small Cell Lung |
NCT01469000 |
| Gefitinib in Treating Patients With Recurrent or Metastatic Esophageal or Gastroesophageal Junction Cancer |
Completed |
Esophageal Cancer |
NCT00268346 |
| Gefitinib in Treating Patients With Advanced Unresectable Hepatocellular Carcinoma (Liver Cancer) |
Completed |
Adult Primary Hepatocellular Carcinoma|Advanced Adult Primary Liver Cancer|Localized Unresectable Adult Primary Liver Cancer|Recurrent Adult Primary Liver Cancer |
NCT00071994 |
| Gefitinib With or Without Simvastatin in Non-Small Cell Lung Cancer (NSCLC) |
Completed |
Lung Cancer |
NCT00452244 |
| Study Of SU011248 Plus Gefitinib (Iressa) In Patients With Advanced Renal Cell Carcinoma |
Completed |
Carcinoma, Renal Cell |
NCT00113529 |
| Targeted Therapy With Gefitinib in Patients With USP8-mutated Cushing's Disease |
Unknown status |
Cushing's Disease|Corticotrophin Adenoma |
NCT02484755 |
| Phase 2 Study Of Neoadjuvant Iressa Treatment In Stage 1 NSCLC |
Completed |
LUNG CANCER |
NCT00188617 |
| Study to Evaluate the Safety and Efficacy of Gefitinib, in Subjects With EFGR Amplification Refractory Solid Tumors |
Recruiting |
Solid Tumor |
NCT02447419 |
| Topotecan and Gefitinib (Iressa) for Ovarian, Peritoneal, or Fallopian Tube Cancer |
Active, not recruiting |
Ovarian Cancer|Peritoneal Neoplasms|Fallopian Tube Cancer |
NCT00317772 |
| A Feasibility Study With Iressa in Resistant Cytokeratin-Positive Tumor Cells Circulating in the Blood of Women With Breast Cancer |
Completed |
Breast Cancer |
NCT00428896 |
| Combination of Chemotherapy and Gefitinib as First-line Treatment |
Completed |
Non-Small-Cell Lung Cancer |
NCT02148380 |
| Comparator-Controlled Study for EGFR(+) Patients With Multiple BMs From NSCLC (BROKE) (EGFR-epidermal Growth Factor Receptor;BM-brain Metastases) |
Unknown status |
Non-Small Cell Lung Cancer|Brain Metastases|EGFR Gene Mutation |
NCT02338011 |
| Iressa Follow-up Trial |
Completed |
Lung Cancer|Breast Cancer |
NCT00357734 |
| A Phase I Study of Safety and Pharmacokinetics of Volitinib in Combination With Gefitinib in EGFR(+) NSCLC |
Active, not recruiting |
Non-Small Cell Lung Cancer |
NCT02374645 |
| Intercalating and Maintenance Gefitinib in Combination With Chemotherapy for Advanced EGFR-mutant NSCLC |
Terminated |
Non-small Cell Lung Cancer Metastatic|EGFR Activating Mutation |
NCT02299765 |
| Pemetrexed/Cisplatin Intercalating Gefitinib Treating EGFR Wild NSCLC |
Recruiting |
Non Small Cell Lung Cancer |
NCT03374280 |
| Phase II Trial to Correlate Radiographic Response Induced By Gefitinib With Mutations in the Protein-Tyrosine Kinase Domain of the EGF Receptor Gene |
Completed |
Lung Cancer|Non-small Cell Lung Cancer|Bronchioloalveolar Cancer |
NCT00588445 |
| Gefitinib in Treating Children With Refractory Solid Tumors |
Completed |
Unspecified Childhood Solid Tumor, Protocol Specific |
NCT00040781 |
| Gefitinib Usage and Outcomes in Routine Treatment |
Completed |
Lung Cancer |
NCT01818947 |
| Study Evaluating Gefitinib (IRESSA®) in Subjects With Solid Malignancies That Are Locally Advanced, Recurrent or Metastatic |
Completed |
Tumors |
NCT00127829 |
| Alflutinib Mesylate Versus Gefitinib in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer ďĽFLAG) |
Active, not recruiting |
Locally Advanced or Metastatic EGFR Sensitising Mutation Positive Non-small Cell Lung Cancer |
NCT03787992 |
| Gefitinib in Treating Patients With Metastatic or Unresectable Head and Neck Cancer or Non-Small Cell Lung Cancer |
Completed |
Anaplastic Thyroid Cancer|Insular Thyroid Cancer|Metastatic Parathyroid Cancer|Recurrent Adenoid Cystic Carcinoma of the Oral Cavity|Recurrent Basal Cell Carcinoma of the Lip|Recurrent Esthesioneuroblastoma of the Paranasal Sinus and Nasal Cavity|Recurrent Inverted Papilloma of the Paranasal Sinus and Nasal Cavity|Recurrent Lymphoepithelioma of the Nasopharynx|Recurrent Lymphoepithelioma of the Oropharynx|Recurrent Metastatic Squamous Neck Cancer With Occult Primary|Recurrent Midline Lethal Granuloma of the Paranasal Sinus and Nasal Cavity|Recurrent Mucoepidermoid Carcinoma of the Oral Cavity|Recurrent Non-small Cell Lung Cancer|Recurrent Parathyroid Cancer|Recurrent Salivary Gland Cancer|Recurrent Squamous Cell Carcinoma of the Hypopharynx|Recurrent Squamous Cell Carcinoma of the Larynx|Recurrent Squamous Cell Carcinoma of the Lip and Oral Cavity|Recurrent Squamous Cell Carcinoma of the Nasopharynx|Recurrent Squamous Cell Carcinoma of the Oropharynx|Recurrent Squamous Cell Carcinoma of the Paranasal Sinus and Nasal Cavity|Recurrent Thyroid Cancer|Recurrent Verrucous Carcinoma of the Larynx|Stage III Follicular Thyroid Cancer|Stage III Papillary Thyroid Cancer|Stage III Salivary Gland Cancer|Stage III Squamous Cell Carcinoma of the Hypopharynx|Stage III Squamous Cell Carcinoma of the Larynx|Stage III Verrucous Carcinoma of the Larynx|Stage IIIB Non-small Cell Lung Cancer|Stage IV Lymphoepithelioma of the Nasopharynx|Stage IV Non-small Cell Lung Cancer|Stage IV Squamous Cell Carcinoma of the Hypopharynx|Stage IV Squamous Cell Carcinoma of the Nasopharynx|Stage IVA Adenoid Cystic Carcinoma of the Oral Cavity|Stage IVA Basal Cell Carcinoma of the Lip|Stage IVA Esthesioneuroblastoma of the Paranasal Sinus and Nasal Cavity|Stage IVA Follicular Thyroid Cancer|Stage IVA Inverted Papilloma of the Paranasal Sinus and Nasal Cavity|Stage IVA Lymphoepithelioma of the Oropharynx|Stage IVA Midline Lethal Granuloma of the Paranasal Sinus and Nasal Cavity|Stage IVA Mucoepidermoid Carcinoma of the Oral Cavity|Stage IVA Papillary Thyroid Cancer|Stage IVA Salivary Gland Cancer|Stage IVA Squamous Cell Carcinoma of the Larynx|Stage IVA Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage IVA Squamous Cell Carcinoma of the Oropharynx|Stage IVA Squamous Cell Carcinoma of the Paranasal Sinus and Nasal Cavity|Stage IVA Verrucous Carcinoma of the Larynx|Stage IVA Verrucous Carcinoma of the Oral Cavity|Stage IVB Adenoid Cystic Carcinoma of the Oral Cavity|Stage IVB Basal Cell Carcinoma of the Lip|Stage IVB Esthesioneuroblastoma of the Paranasal Sinus and Nasal Cavity|Stage IVB Follicular Thyroid Cancer|Stage IVB Inverted Papilloma of the Paranasal Sinus and Nasal Cavity|Stage IVB Lymphoepithelioma of the Oropharynx|Stage IVB Midline Lethal Granuloma of the Paranasal Sinus and Nasal Cavity|Stage IVB Mucoepidermoid Carcinoma of the Oral Cavity|Stage IVB Papillary Thyroid Cancer|Stage IVB Salivary Gland Cancer|Stage IVB Squamous Cell Carcinoma of the Larynx|Stage IVB Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage IVB Squamous Cell Carcinoma of the Oropharynx|Stage IVB Squamous Cell Carcinoma of the Paranasal Sinus and Nasal Cavity|Stage IVB Verrucous Carcinoma of the Larynx|Stage IVB Verrucous Carcinoma of the Oral Cavity|Stage IVC Adenoid Cystic Carcinoma of the Oral Cavity|Stage IVC Basal Cell Carcinoma of the Lip|Stage IVC Esthesioneuroblastoma of the Paranasal Sinus and Nasal Cavity|Stage IVC Follicular Thyroid Cancer|Stage IVC Inverted Papilloma of the Paranasal Sinus and Nasal Cavity|Stage IVC Lymphoepithelioma of the Oropharynx|Stage IVC Midline Lethal Granuloma of the Paranasal Sinus and Nasal Cavity|Stage IVC Mucoepidermoid Carcinoma of the Oral Cavity|Stage IVC Papillary Thyroid Cancer|Stage IVC Salivary Gland Cancer|Stage IVC Squamous Cell Carcinoma of the Larynx|Stage IVC Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage IVC Squamous Cell Carcinoma of the Oropharynx|Stage IVC Squamous Cell Carcinoma of the Paranasal Sinus and Nasal Cavity|Stage IVC Verrucous Carcinoma of the Larynx|Stage IVC Verrucous Carcinoma of the Oral Cavity|Thryoid Gland Nonmedullary Carcinoma|Thyroid Gland Medullary Carcinoma|Tongue Cancer|Untreated Metastatic Squamous Neck Cancer With Occult Primary |
NCT00068497 |
| LUX-Lung 7: A Phase IIb Trial of Afatinib(BIBW2992) Versus Gefitinib for the Treatment of 1st Line EGFR Mutation Positive Adenocarcinoma of the Lung |
Completed |
Lung Neoplasms |
NCT01466660 |
| Iressa for EGFR Mutation Negative Non-small Cell Lung Cancer (NSCLC) |
Unknown status |
Nonsmall Cell Lung Cancer |
NCT01312337 |
| A Phase 1b/2 Study in Asian Subjects With Non-Small Cell Lung Cancer |
Completed |
Carcinoma, Non-Small Cell-Lung|Lung Neoplasms|Lung Cancer|Respiratory Tract Neoplasms |
NCT01039948 |
| Neoadjuvant IRESSA As Single Agent PreopTherapy for NSCLC With Molecular Correlates |
Terminated |
Lung Cancer |
NCT00104728 |
| Clinical Value of Sequential Gefitinib With Pemetrexed/Platinum for Advanced NSCLC |
Completed |
NSCLC |
NCT01769066 |
| Gefitinib in Treating Patients Who Are Undergoing Surgery and/or Radiation Therapy for Locally Advanced or Recurrent Squamous Cell Skin Cancer |
Completed |
Recurrent Skin Cancer|Squamous Cell Carcinoma of the Skin |
NCT00126555 |
| Combination of Metformin With Gefitinib to Treat NSCLC |
Completed |
NSCLC|EGFR Gene Amplification|Advanced Cancer|Stage IIIB NSCLC|Stage IV NSCLC |
NCT01864681 |
| MEDI4736 (Anti PD-L1) Combined With Gefitinib in Subjects With Non-Small Cell Lung Cancer(NSCLC). |
Active, not recruiting |
Carcinoma, Non-Small-Cell Lung |
NCT02088112 |
| First Line IRESSA™ Versus Carboplatin/Paclitaxel in Asia |
Completed |
Non-Small Cell Lung Cancer |
NCT00322452 |
| Arimidex/Faslodex/Iressa Study: A Trial Using Arimidex, Faslodex and Iressa in Women With Breast Cancer |
Terminated |
BREAST CANCER |
NCT00206414 |
| Gefitinib in Treating Patients With Persistent or Recurrent Endometrial Cancer |
Completed |
Recurrent Uterine Corpus Carcinoma |
NCT00027690 |
| Clinical Study of YYJD Decoction Combined With Gefitinib in Advanced Pulmonary Adenocarcinoma |
Enrolling by invitation |
Cancer |
NCT02929693 |
| A Phase II Study of 250-mg ZD1839 Monotherapy in Recurrent or Metastatic or Both Recurrent and Metastatic Squamous Cell Carcinoma |
Completed |
Head and Neck Cancer |
NCT01185158 |
| A Phase III, Randomized, Multi-center Study to Determine the Efficacy of the Intercalating Combination Treatment of Chemotherapy and Gefitinib or Chemotherapy as Adjuvant Treatment in NSCLC With Common EGFR Mutations. |
Recruiting |
Completely Resected NSCLC With Common EGFR Mutations |
NCT03381066 |
| Gefitinib in Treating Patients With Malignant Mesothelioma |
Completed |
Advanced Malignant Mesothelioma|Epithelial Mesothelioma|Recurrent Malignant Mesothelioma|Sarcomatous Mesothelioma |
NCT00025207 |
| A Study of ASP8273 vs. Erlotinib or Gefitinib in First-line Treatment of Patients With Stage IIIB/IV Non-small Cell Lung Cancer Tumors With EGFR Activating Mutations |
Terminated |
Non-small Cell Lung Cancer (NSCLC) |
NCT02588261 |
| Pharmocokinetic/Pharmacodynamic (PK/PD) Study of the Combination Cetuximab/Gefitinib |
Completed |
Colorectal Cancer|Head and Neck Cancer|Non Small Cell Lung Cancer (NSCLC) |
NCT00820417 |
| Gefitinib and Radiation Therapy in Treating Children With Newly Diagnosed Gliomas |
Completed |
Untreated Childhood Anaplastic Astrocytoma|Untreated Childhood Anaplastic Oligodendroglioma|Untreated Childhood Brain Stem Glioma|Untreated Childhood Giant Cell Glioblastoma|Untreated Childhood Glioblastoma|Untreated Childhood Gliomatosis Cerebri|Untreated Childhood Gliosarcoma|Untreated Childhood Oligodendroglioma |
NCT00042991 |
| Gefitinib in Treating Patients With Locally Advanced or Metastatic Thyroid Cancer That Did Not Respond to Iodine Therapy |
Completed |
Head and Neck Cancer |
NCT00095836 |
| Gefitinib and Radiation Therapy in Treating Patients With Glioblastoma Multiforme |
Completed |
Adult Giant Cell Glioblastoma|Adult Glioblastoma|Adult Gliosarcoma |
NCT00052208 |
| Gefitinib in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer |
Completed |
Bronchoalveolar Cell Lung Cancer|Recurrent Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer |
NCT00029003 |
| Study of Gefitinib and Docetaxel as Salvage Therapy in Advanced Pancreatic Carcinoma |
Completed |
Cancer |
NCT00177242 |
| Gefitinib and Everolimus in Treating Patients With Stage IIIB or Stage IV or Recurrent Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00096486 |
| Clinical Trial of Neoadjuvant Targeted Treatment to NSCLC Patients |
Not yet recruiting |
2-year Disease-Free Survival |
NCT03203590 |
| A Study of SH-1028 Tablets Versus Gefitinib in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer |
Not yet recruiting |
Non-Small Cell Lung Cancer |
NCT04239833 |
| Open Label Extension Study With Gefitinib (IRESSA™) for Completing Trial Patients Who May Benefit From Further Treatment |
Approved for marketing |
Non Small Cell Lung Cancer (NSCLC) |
NCT00683306 |
| Gefitinib Combined With Thalidomide to Treat NSCLC |
Unknown status |
NSCLC |
NCT02387086 |
| Phase III Study of Nazartinib (EGF816) Versus Erlotinib/Gefitinib in First-line Locally Advanced / Metastatic NSCLC With EGFR Activating Mutations |
Withdrawn |
Carcinoma, Non-small Cell Lung |
NCT03529084 |
| Trastuzumab and Gefitinib in Treating Patients With Metastatic Breast Cancer |
Completed |
Male Breast Cancer|Recurrent Breast Cancer|Stage IV Breast Cancer |
NCT00024154 |
| Study of Pemetrexed Versus Gefitinib in Patients With Locally Advanced or Metastatic Non Small Cell Lung Cancer Who Have Previously Received Platinum-Based Chemotherapy Without Epidermal Growth Factor Receptor (EGFR) Mutations |
Completed |
Non Small Cell Lung Cancer |
NCT00891579 |
| First-line Treatment for Adenocarcinoma Patients With Epidermal Growth Factor Receptor (EGFR) Mutation |
Completed |
Pulmonary Cancer |
NCT00344773 |
| Gefitinib in Treating Patients With Previously Untreated Stage IIIB or Stage IV Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00411047 |
| Comparing Anastrozole-Placebo to the Combination Anastrozole-ZD1839 in Postmenopausal Patients With Estrogen Receptor and/or Progesterone Receptor Metastatic Breast Cancer |
Completed |
Breast Cancer |
NCT00077025 |
| Erlotinib 100mg qd Versus Gefitinib 250mg qd for EGFR Mutant Nsclc |
Unknown status |
Advanced Stage Non Small Cell Lung Cancer |
NCT01955421 |
| Study to Assess Safety/Tolerability/Efficacy of Gefitinib Versus Docetaxel in Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) |
Terminated |
Carcinoma, Non-Small-Cell Lung |
NCT00536107 |
| Randomized Gefitinib Trial |
Completed |
Non Small Cell Lung Cancer |
NCT00807066 |
| Gefitinib in Treating Patients With Locally Advanced Esophageal Cancer |
Completed |
Esophageal Cancer |
NCT00100945 |
| Iressa v BSC (Best Supportive Care) in First Line NSCLC |
Completed |
NSCLC |
NCT00259064 |
| Trial of Induction Chemotherapy With Carboplatin and Paclitaxel, Followed by Concurrent Chemotherapy/Radiation Therapy With ZD1839 (IRESSA), 5-FU, Hydroxyurea, and Twice-Daily Radiation, Followed by Adjuvant ZD1839 Monotherapy in Patients With Locally Advanced Head & Neck Cancer |
Unknown status |
Head and Neck Cancer |
NCT01185171 |
| Selumetinib in Combination With Gefitinib in NSCLC Patients |
Completed |
Non-small Cell Lung Cancer (NSCLC) |
NCT02025114 |
| An Open-label, Phase II Trial of ZD1839 (IRESSA) in Patients With Malignant Mesothelioma |
Completed |
Mesothelioma |
NCT00787410 |
| Iressa as Second Line Therapy in Advanced NSCLC-Asia |
Completed |
NSCLC |
NCT00478049 |
| Feasibility Study to Estimate Number of Patients With Precancerous Areas in Their Airways and the Response to Gefitinib |
Terminated |
Lung Cancer|Head and Neck Cancer |
NCT01405846 |
| To Evaluate the Efficacy and Safety of Gefitinib in Adjuvant Chemotherapy for Lung Adenocarcinoma |
Recruiting |
NSCLC|EGFR |
NCT03656393 |
| Paclitaxel/Carboplatin (PC) Followed by Gefitinib Versus PC in Advanced Non-small Cell Lung Cancer |
Completed |
Non Small Cell Lung Cancer |
NCT01196234 |
| Gefitinib and PEG-Interferon Alfa-2b in Treating Patients With Unresectable or Metastatic Kidney Cancer |
Terminated |
Kidney Cancer |
NCT00467077 |
| First-line Gefitinib Versus Chemotherapy for Lung Adenocarcinoma in Never Smoker |
Completed |
Lung Cancer |
NCT00455936 |
| Gefitinib and Celecoxib in Treating Patients With Refractory Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00068653 |
| ZD 1839 in Treating Patients With Metastatic or Recurrent Cancer of the Head and Neck |
Completed |
Head and Neck Cancer |
NCT00015964 |
| Phase I Study of Iressa and CRT/IMRT in Chinese Patients With IIIB/IV NSCLC After Failure of Platinum-Based Chemotherapy |
Unknown status |
Carcinoma, Non-Small-Cell Lung |
NCT00497250 |
| Trial of ZD1839 (Iressa) and Tamoxifen in Breast Cancer Patients |
Terminated |
Breast Cancer |
NCT00206492 |
| Pemetrexed (ALIMTA) and Gefitinib (IRESSA®) in Never-Smoker and Adenocarcinoma Patients With Non-Small Cell Lung Cancer Previously Treated With Platinum-Based Chemotherapy |
Unknown status |
Non-small Cell Lung Cancer |
NCT01066195 |
| Oxaliplatin With or Without Gefitinib in Treating Patients With Metastatic or Locally Recurrent Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00026299 |
| IRESSA™ (Gefitinib) in Breast Cancer Patients |
Completed |
Breast Cancer |
NCT00632723 |
| Study of Vorinostat and Gefitinib in Relapsed/ or Refractory Patients With Advanced Non-small Cell Carcinoma (NSCLC) |
Unknown status |
Non-Small-Cell Lung Carcinoma |
NCT01027676 |
| Trial of Iressa in Prostate Cancer Patients |
Completed |
Prostate Cancer |
NCT00265070 |
| Gefitinib in Treating Patients With Progressive Metastatic Neuroendocrine Tumors |
Completed |
Gastrinoma|Glucagonoma|Insulinoma|Metastatic Gastrointestinal Carcinoid Tumor|Pancreatic Polypeptide Tumor|Recurrent Gastrointestinal Carcinoid Tumor|Recurrent Islet Cell Carcinoma|Somatostatinoma|WDHA Syndrome |
NCT00075439 |
| ZD1839 (Iressa) for Recurrent or Metastatic Squamous Cell Carcinoma of the Skin |
Completed |
Skin Cancer |
NCT00054691 |
| Phase III Trial Of Docetaxel Versus Docetaxel Plus ZD1839 In Head And Neck Cancer |
Terminated |
Recurrent Head and Neck Cancer|Metastatic Head and Neck Cancer |
NCT00088907 |
| SELINE: Second-Line Iressa Phase IV Study in NSCLC Patients |
Completed |
Non Small Cell Lung Carcinoma |
NCT00608868 |
| Clinical Trial of YH25448(Lazertinib) as the First-line Treatment in Patients With EGFR Mutation Positive Locally Advanced or Metastatic NSCLC (LASER301) |
Recruiting |
Non-Small Cell Lung Cancer |
NCT04248829 |
| Ph II Early BC Pre-Surgical Biologic Study |
Completed |
Breast Cancer |
NCT00637026 |
| Adjuvant Therapy of Gefitinib (Iressa, ZD1839) in Patients With Resectable Hepatocellular Carcinoma |
Active, not recruiting |
Liver Cancer |
NCT00282100 |
| A Safety and Efficacy Study of INC280 and Gefitinib in Patients With EGFR Mutated, c-MET-amplified NSCLC Who Have Progressed After EGFRi Treatment |
Active, not recruiting |
Non-small Cell Lung Cancer |
NCT01610336 |
| Phase II Metastatic ER+/PgR+ Nolvadex +/- Iressa Study |
Completed |
Breast Neoplasms |
NCT00229697 |
| Docetaxel in Combination With Iressa in Previously Treated Patients With Pancreatic Cancer |
Completed |
Metastatic Pancreatic Carcinoma |
NCT00137761 |
| Everolimus and Gefitinib in Treating Patients With Progressive Glioblastoma Multiforme or Progressive Metastatic Prostate Cancer |
Completed |
Brain and Central Nervous System Tumors|Prostate Cancer |
NCT00085566 |
| Gefitinib Followed By Surgery in Treating Women With Ductal Carcinoma In Situ of the Breast |
Terminated |
Breast Cancer |
NCT00082667 |
| Phase II Study of Docetaxel + ZD1839 in Elderly Patients With Non-Small Cell Lung Cancer |
Completed |
Non-Small Cell Lung Cancer |
NCT00231465 |
| Assess the Efficacy of Whole Brain Radiation Therapy in Lung Cancer Patients With Brain Metastasis |
Terminated |
EGFR-mutated Lung Adenocarcinoma|Brain Metastasis |
NCT01363557 |
| Gefitinib for EGFR Sensitive Mutation Postoperative Stage Ib NSCLC Patients |
Terminated |
NSCLC |
NCT02526537 |
| Induction Therapy With Intercalated Tyrosine Kinase Inhibitor (TKI) and Chemotherapy in NSCLC With Activating Epidermal Growth Factor Receptor (EGFR) Mutation in Stages II-IIIB |
Terminated |
Non-squamous Non-small Cell Lung Cancer Stage II|Non-squamous Non-small Cell Lung Cancer Stage IIIA|Non-squamous Non-small Cell Lung Cancer Stage IIIB|Activating EGFR Mutation|NSCLC |
NCT02326285 |
| Gefitinib in Treating Patients With Stage IB, II, or IIIA Non-small Cell Lung Cancer That Was Completely Removed by Surgery |
Completed |
Adenocarcinoma of the Lung|Adenosquamous Cell Lung Cancer|Bronchoalveolar Cell Lung Cancer|Large Cell Lung Cancer|Squamous Cell Lung Cancer|Stage IB Non-small Cell Lung Cancer|Stage IIA Non-small Cell Lung Cancer|Stage IIB Non-small Cell Lung Cancer|Stage IIIA Non-small Cell Lung Cancer |
NCT00049543 |
| EGFR Mutation Positive NSCLC Patients With Gefitinib and Thalidomide |
Recruiting |
NSCLC Stage IV|Chemotherapy Effect |
NCT03341494 |
| Iressa Re-challenge in Advanced NSCLC EGFR-mutated Patients |
Terminated |
Lung Cancer |
NCT02025218 |
| Intercalated Administration of PamCis With Gefitinib or Placebo as First Line Lung Adenocarcinoma in Never Smokers |
Unknown status |
Non Small Cell Lung Cancer|Adenocarcinoma |
NCT01502202 |
| Cediranib Maleate With or Without Gefitinib in Treating Patients With Recurrent or Progressive Glioblastoma |
Terminated |
Glioblastoma |
NCT01310855 |
| Gefitinib in Treating Patients With Newly Diagnosed Glioblastoma Multiforme |
Completed |
Adult Giant Cell Glioblastoma|Adult Glioblastoma|Adult Gliosarcoma |
NCT00014170 |
| Gefitinib and Capecitabine in Treating Patients With Advanced Solid Tumors |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00039390 |
| Iressa/Docetaxel in Non-Small-Cell Lung Cancer |
Withdrawn |
Non-small Cell Lung Cancer |
NCT00048087 |
| Effect of Iressa With/Without Concurrent Chemoradiotherapy on Tumor Gene Expression Profiles in Patients With Advanced Non-Nasopharyngeal Head and Neck Carcinoma |
Completed |
Head and Neck Cancer |
NCT00228488 |
| Open Label Trial to Assess Iressa in Prostate Cancer Patients |
Completed |
Prostate Cancer |
NCT00635856 |
| Therapy With Docetaxel and ZD1839 for Patients Who Have Advanced Breast Cancer |
Completed |
Breast Neoplasms |
NCT00052169 |
| Gefitinib in Treating Patients With Recurrent and/or Metastatic Head and Neck Cancer |
Completed |
Head and Neck Cancer |
NCT00024089 |
| Gefitinib in Combination With Chemoradiation in Resectable Gastric Cancer |
Terminated |
Subjects With Resectable Local or Locally Advanced, Non-Metastatic (T2-T4, N0-N3, M0; Stages II and III) and Histologically-Confirmed Intestinal GC |
NCT00237900 |
| Gefitinib Plus Combination Chemotherapy in Treating Patients With Locally Advanced or Metastatic Bladder Cancer |
Completed |
Metastatic Transitional Cell Cancer of the Renal Pelvis and Ureter|Recurrent Transitional Cell Cancer of the Renal Pelvis and Ureter |
NCT00041106 |
| Gefitinib in Treating Patients With Esophageal Cancer That is Progressing After Chemotherapy |
Completed |
Adenocarcinoma of the Gastroesophageal Junction|Esophageal Cancer |
NCT01243398 |
| UFUR (Tegafur/Uracil) Plus Iressa in Non-small-cell Lung Cancer |
Completed |
Non-Small-Cell Lung Cancer |
NCT01037998 |
| A Phase I Study of Cetuximab in Combination With Gefitinib in Patients With Advanced/Metastatic Non-Small Cell Lung Cancer |
Completed |
Non-Small-Cell Lung Carcinoma |
NCT00162318 |
| Erlotinib Re-Challenge for Recurrent EGFR-mutant Lung Cancer in Patients Who Previously Received Adjuvant Erlotinib or Gefitinib |
Terminated |
Lung Cancer |
NCT01189435 |
| Open-Label Extension of Other SZ1839 (Iressa) Trials |
Completed |
Cancer |
NCT00635973 |
| Safety and Efficacy Study of Icotinb in Non-small Cell Lung Cancer (NSCLC) Patients |
Completed |
Non-small Cell Lung Cancer |
NCT01040780 |
| S0031, ZD 1839 in Treating Patients With Advanced Cancer of the Urinary Tract |
Completed |
Bladder Cancer|Transitional Cell Cancer of the Renal Pelvis and Ureter|Urethral Cancer |
NCT00014144 |
| Iressa Expanded Access Program (EAP) |
Completed |
Carcinoma|Non-small-cell Lung|Metastases|Neoplasm |
NCT00034879 |
| Tepotinib With Gefitinib in Subjects With Locally Advanced or Metastatic Non-small Cell Lung Cancer (NSCLC) |
Active, not recruiting |
Non-small Cell Lung Cancer |
NCT01982955 |
| Phase II Trial of Preoperative Therapy With Gefitinib and Chemotherapy in Patients With ERneg Breast Cancer |
Completed |
Breast Cancer |
NCT00239343 |
| Gefitinib Long-term Survivor Study |
Completed |
EGFR Mutation Positive Advanced Non-small-cell Lung Cancer |
NCT02932345 |
| Gefitinib and Radiation Therapy With or Without Cisplatin in Treating Patients With Stage III or Stage IV Head and Neck Cancer |
Terminated |
Stage III Squamous Cell Carcinoma of the Hypopharynx|Stage III Squamous Cell Carcinoma of the Larynx|Stage III Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage III Squamous Cell Carcinoma of the Oropharynx|Stage IV Squamous Cell Carcinoma of the Hypopharynx|Stage IV Squamous Cell Carcinoma of the Larynx|Stage IV Squamous Cell Carcinoma of the Lip and Oral Cavity|Stage IV Squamous Cell Carcinoma of the Oropharynx |
NCT00033449 |
| Head and Neck Phase III Iressa Versus Methotrexate Refractory: Iressa Versus Methotrexate (IMEX) |
Completed |
Squamous Cell Carcinoma of the Head and Neck |
NCT00206219 |
| A Study to Predict Gefitinib' s Efficacy for Lung Cancer by Plasma Free Nucleic Acids EGFR Gene Mutation Test |
Unknown status |
Lung Adenocarcinoma |
NCT02738684 |
| Gefitinib and Combination Chemotherapy in Treating Patients With Advanced or Recurrent Colorectal Cancer |
Terminated |
Adenocarcinoma of the Colon|Adenocarcinoma of the Rectum|Mucinous Adenocarcinoma of the Colon|Mucinous Adenocarcinoma of the Rectum|Recurrent Colon Cancer|Recurrent Rectal Cancer|Signet Ring Adenocarcinoma of the Colon|Signet Ring Adenocarcinoma of the Rectum|Stage IV Colon Cancer|Stage IV Rectal Cancer |
NCT00052585 |
| Paclitaxel, Cisplatin, Gefitinib, and Radiation Therapy Followed by Surgery and Gefitinib in Treating Patients With Locally Advanced Cancer of the Esophagus or Gastroesophageal Junction That Can Be Removed By Surgery |
Terminated |
Esophageal Cancer |
NCT00493025 |
| IRESSA™ (Gefitinib) With Cisplatin Plus Radiotherapy for the Treatment of Previously Untreated Unresected Late Stage III/IV Non-Metastatic Head and Neck Squamous Cell Carcinoma |
Completed |
Neoplasms, Squamous Cell |
NCT00229723 |
| A Study of Alimta/Cisplatin/Gefitinib for Asian Non-smoking Participants With Non Small Cell Lung Cancer |
Completed |
Non Small Cell Lung Cancer |
NCT01017874 |
| A Study of IRESSA Treatment Beyond Progression in Addition to Chemotherapy Versus Chemotherapy Alone |
Completed |
Non-Small Cell Lung Cancer |
NCT01544179 |
| Iressa vs Best Supportive Care - 2nd/3rd Line Survival Study |
Completed |
NSCLC |
NCT00242801 |
| BCG With or Without Gefitinib in Treating Patients With High-Risk Bladder Cancer |
Terminated |
Bladder Cancer |
NCT00352079 |
| Gefitinib With or Without Carboplatin and Paclitaxel in Treating Older Patients With Unresectable or Metastatic Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00062062 |
| Prophylactic Cranial Irradiation in Erlotinib/Gefitinib-responders With Non-small Cell Lung Cancer (NSCLC) (RT1001) |
Unknown status |
Non-small Cell Lung Cancer |
NCT01158170 |
| Cisplatin, Fluorouracil, Iressa, and Radiation Therapy Patients With Locally Advanced Head and Neck Cancer |
Completed |
Head and Neck Cancer |
NCT00352105 |
| Gefitinib and PEG-Interferon Alfa-2a in Treating Patients With Unresectable or Metastatic Skin Cancer |
Unknown status |
Non-melanomatous Skin Cancer |
NCT00423397 |
| Phase II Gemcitabine + Cisplatin +/- Iressa Bladder CCT |
Completed |
Bladder Cancer |
NCT00246974 |
| Post-operative Radiotherapy With Cisplatin Alone or in Combination With Iressa in Upper Aerodigestive Tract Carcinomas |
Terminated |
Head and Neck Cancer |
NCT00169221 |
| Combination Chemotherapy, Radiation Therapy, and Gefitinib in Treating Patients With Stage III Non-Small Cell Lung Cancer |
Completed |
Adenocarcinoma of the Lung|Adenosquamous Cell Lung Cancer|Bronchoalveolar Cell Lung Cancer|Large Cell Lung Cancer|Squamous Cell Lung Cancer|Stage IIIA Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer |
NCT00040794 |
| Anastrozole With or Without Gefitinib in Treating Postmenopausal Women With Metastatic or Locally Recurrent Breast Cancer |
Completed |
Breast Cancer |
NCT00066378 |
| Genius Study Study to Compare Efficacy and Safety of Gefitinib/ Pemetrexed With Pemetrexed Alone as Maintenance Therapy in Patients With Stage IV EGFR Mutation Negative or T790M Single Mutation Who Respond to Pemetrexed/ Platinum as First-line Therapy |
Unknown status |
NSCLC |
NCT01579630 |
| Study of Gefitinib Compared With Pemetrexed/Cisplatin in Advanced Non-Small Cell Lung Cancer Patients |
Unknown status |
Toxicity|Non-small Cell Lung Cancer |
NCT01192243 |
| ZD1839 With Hypofractionated Radiation Therapy With an Immobilization Device for Advanced Non-Small Cell Lung Cancer |
Completed |
Non-Small Cell Lung Carcinoma (NSCLC) |
NCT00328562 |
| Gefitinib in the Treatment of the First Relapse of Prostate Cancer Beyond Prostatectomy or Radiotherapy |
Completed |
Prostate Cancer |
NCT00241475 |
| Anastrozole and ZD1839 Compared With Fulvestrant and ZD1839 in Postmenopausal Women w/ Metastatic Breast Cancer |
Completed |
Estrogen Receptor-positive Breast Cancer|Progesterone Receptor-positive Breast Cancer|Recurrent Breast Cancer|Stage IV Breast Cancer |
NCT00057941 |
| Phase II Iressa Versus Vinorelbine (INVITE) |
Completed |
Non-Small-Cell Lung Carcinoma |
NCT00256711 |
| Iressa as a First-Line Treatment in Chemonaive Patients With Inoperable Non-Small Cell Lung Cancer |
Completed |
Non-Small Cell Lung Cancer |
NCT00173875 |
| Gefitinib After Chemotherapy in Treating Patients With Stage IIIB or Stage IV Non-Small Cell Lung Cancer |
Terminated |
Lung Cancer |
NCT00091156 |
| Radiation Therapy Combined With Either Gefitinib or Temozolomide in Pats With NSCLC and Brain Metastases |
Completed |
Lung Cancer|Metastatic Cancer |
NCT00238251 |
| A Phase II Exploratory, Multicentre, Open-label, Non-comparative Study of ZD1839 (Iressa) and Radiotherapy in the Treatment of Patients With Glioblastoma Multiforme |
Completed |
Glioblastoma |
NCT00238797 |
| Gefitinib in Treating Patients With Recurrent or Progressive CNS Tumors |
Completed |
Brain and Central Nervous System Tumors |
NCT00025675 |
| Gefitinib in Treating Patients With Recurrent Metastatic Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00025350 |
| Gefitinib, Trastuzumab, and Docetaxel in Treating Patients With Metastatic Breast Cancer |
Completed |
Breast Cancer |
NCT00086957 |
| Gefitinib in Treating Patients With Locally Advanced or Metastatic Synovial Sarcoma |
Completed |
Sarcoma |
NCT00052754 |
| A Study of ZD1839 Effects on Cell Proliferation in Breast Cancer |
Withdrawn |
Breast Cancer |
NCT00252811 |
| Iressa Versus Docetaxel (Taxotere) |
Completed |
Non-Small-Cell Lung Carcinoma |
NCT00076388 |
| Gefitinib in Treating Patients With Recurrent or Persistent Ovarian Epithelial Cancer or Primary Peritoneal Cancer |
Completed |
Ovarian Cancer|Primary Peritoneal Cavity Cancer |
NCT00023699 |
| Phase I Study of AZD2171 co-Administered With Fixed Multiple Oral Doses of ZD1839 in Patients With Advanced Cancer |
Completed |
Advanced Tumor |
NCT00502060 |
| A Study of IRESSA in Relapsed and Refractory Small Cell Lung Cancer |
Completed |
Small Cell Lung Cancer |
NCT00298688 |
| A Trial to Evaluate the Combination of Iressa & Faslodex® in Patients With Advanced or Metastatic Breast Cancer |
Completed |
Breast Cancer |
NCT00234403 |
| A Study to Evaluate Safety and Efficacy of HS-10296 as First-Line Treatment in Patients |
Recruiting |
Non Small Cell Lung Cancer |
NCT03849768 |
| An Expanded Access Programme With Iressa for Patients With Non-Small-Cell Lung Cancer and Cancer of the Head and Neck |
Approved for marketing |
Non Small Cell Lung Cancer|Cancer of the Head and Neck |
NCT00684385 |
| Gefitinib in Treating Patients With Stage IIIA Non-Small Cell Lung Cancer |
Unknown status |
Lung Cancer |
NCT00616499 |
| Iressa and Taxotere Study in Patients With Metastatic Urothelial Cancer |
Terminated |
Bladder Cancer |
NCT00479089 |
| A Phase I Study of ZD1839 and Palliative Thoracic Radiotherapy in Patients With Non-small-cell Lung Cancer |
Completed |
Non-small-cell Lung Cancer |
NCT00255489 |
| ZD1839 and Oral Irinotecan in Treating Young Patients With Refractory Solid Tumors |
Completed |
Glioblastoma|Rhabdomyosarcomas|Neuroblastoma|Osteosarcoma |
NCT00132158 |
| Iressa 2nd Line Phase III Study in Japan |
Completed |
Non-Small Cell Lung Cancer |
NCT00252707 |
| Phase II Trial of ZD1839 (Iressa) in Patients With Nonresectable Adrenocortical Carcinoma (ACC) |
Completed |
Nonresectable Adrenocortical Carcinoma |
NCT00215202 |
| Gefitinib as First-Line Therapy Followed by Gemcitabine and Cisplatin in Treating Patients With Stage III or Stage IV Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00217698 |
| Iressa in the Treatment of Brain Metastases From Non Small Cell Lung Cancer |
Terminated |
Brain Neoplasms|Non Small Cell Lung Cancer |
NCT00234442 |
| Gefitinib With or Without Tamoxifen in Treating Patients With Tamoxifen-Resistant Metastatic Breast Cancer |
Completed |
Breast Cancer |
NCT00080743 |
| Second-Line Irinotecan or Gefitinib in Docetaxel Pretreated NSCLC |
Unknown status |
Non-Small Cell Lung Cancer |
NCT00319800 |
| ZD1839 (Iressa™) In Combination With Docetaxel As First-Line Treatment In Patients With Metastatic Breast Cancer |
Completed |
Breast Cancer |
NCT00247481 |
| Observations From Long Term Responders in the Gefitinib (Iressa) Expanded Access Program (EAP) |
Completed |
Non-Small Cell Lung Cancer |
NCT01103089 |
| Study of TLK286 (Telcyta) vs. Gefitinib in Locally Advanced or Metastatic Non-Small Cell Lung Cancer |
Completed |
Non Small Cell Lung Carcinoma |
NCT00080340 |
| Pre- and Postoperative Use of ZD1839 (Iressa) in Recurrent Glioblastoma, Including Translational Research |
Completed |
Recurrent Glioblastoma |
NCT00250887 |
| Gefitinib, Docetaxel, and Radiation Therapy in Treating Patients With Stage III Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00310154 |
| A Randomized Phase III Trial of Chemotherapy Alone Versus Chemotherapy Followed by Gefitinib in Stage IIIB/IV Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00144066 |
| Gefitinib in Treating Patients With Stage I, Stage II, or Stage III Esophageal Cancer That Can Be Removed By Surgery |
Terminated |
Esophageal Cancer |
NCT00258297 |
| Gefitinib in Treating Patients With Metastatic Breast Cancer That Has Progressed After Antiestrogen and Nonsteroidal Aromatase Inhibitor Therapy |
Completed |
Breast Cancer |
NCT00066339 |
| Safety Study to Explore Combination of Gefitinib (ZD1839, Iressa) and Radiotherapy in Non-Metastatic Prostate Cancer |
Completed |
Non-Metastatic Prostate Cancer |
NCT00239291 |
| Gefitinib and Docetaxel in Treating Patients With Advanced Solid Tumors |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00084786 |
| A Phase II Study of Iressa in Patients With Chemo Refractory Germ Cell Tumors Expressing EGFR |
Terminated |
Refractory Germ Cell Tumors Expressing EGRF |
NCT00198159 |
| Management of Advanced Non-Small Cell Lung Cancer (NSCLC) and Clinical Outcomes in Patients Who Received Gefitinib in Thailand |
Completed |
Lung Cancer |
NCT01130961 |
| Gefitinib Plus Temozolomide in Treating Patients With Malignant Primary Glioma |
Completed |
Brain and Central Nervous System Tumors |
NCT00027625 |
| Treatment of Lung Adenocarcinoma With Bronchioloalveolar Feature |
Completed |
Pneumonic-type Adenocarcinoma (P-ADC)|Lung Adenocarcinoma With Bronchiolo-alveolar Feature |
NCT00198380 |
| Phase II Iressa & Carbo/Gem in NSCLC |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00264498 |
| Iressa (ZD1839) Plus Anastrozole (Arimidex) in Patients With Ovarian Cancer |
Completed |
Ovarian Cancer|Peritoneal Carcinoma|Tubal Carcinoma |
NCT00181688 |
| Gefitinib and Sirolimus in Treating Patients With Recurrent or Refractory Stage IIIB or Stage IV Non-Small Cell Lung Cancer |
Withdrawn |
Lung Cancer |
NCT00098462 |
| The IRESSA Novel Head and Neck Chemotherapy Evaluation Study |
Completed |
Squamous Cell Cancer|Cancer of Head and Neck |
NCT00255476 |
| Trial of BMS-690514 in Non-Small Cell Lung Cancer Subjects Who Have Been Treated With Gefitinib or Erlotinib and Are Genotypically EGFR Mutation Positive or Who Have Had a Prior Response |
Completed |
Non-Small-Cell Lung Carcinoma |
NCT01167244 |
| ZD 1839 in Treating Patients With Metastatic Kidney Cancer |
Completed |
Kidney Cancer |
NCT00012337 |
| Gefitinib, Paclitaxel, and Radiation Therapy in Treating Patients With Advanced or Recurrent Squamous Cell Carcinoma of the Head and Neck |
Completed |
Head and Neck Cancer |
NCT00083057 |
| ZD 1839 in Treating Patients With Stage IV or Recurrent Kidney Cancer |
Completed |
Kidney Cancer |
NCT00014183 |
| Gefitinib in Treating Patients With Cervical Cancer |
Completed |
Cervical Cancer|Fallopian Tube Cancer|Ovarian Cancer|Primary Peritoneal Cavity Cancer |
NCT00049556 |
| Neoadjuvant Chemoradiotherapy With or Without Gefitinib in Treating Patients With Stage IIIA or Stage IIIB Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00062270 |
| ZD 1839 in Treating Patients With Glioblastoma Multiforme in First Relapse |
Completed |
Brain and Central Nervous System Tumors |
NCT00016991 |
| Combination Chemotherapy and Radiation Therapy With or Without Gefitinib in Treating Patients With Stage III Non-Small Cell Lung Cancer That Cannot Be Removed By Surgery |
Completed |
Adenocarcinoma of the Lung|Bronchoalveolar Cell Lung Cancer|Large Cell Lung Cancer|Squamous Cell Lung Cancer|Stage IIIA Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer |
NCT00020709 |
| ZD 1839 in Treating Patients With Locally Advanced or Metastatic Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00030524 |
| ZD 1839 in Treating Patients With Prostate Cancer That Has Not Responded to Hormone Therapy |
Completed |
Prostate Cancer |
NCT00025116 |
| A Study of Serum Protein Profiling in Patients With Non-Small Cell Lung Cancer Treated With Gefitinib or Erlotinib |
Completed |
Non Small Cell Lung Cancer |
NCT00717847 |
| Cisplatin and ZD1839 + Re-Irradiation in Recurrent Squamous Cell Cancer of the Head and Neck |
Completed |
Head and Neck Cancer|Carcinoma, Squamous Cell |
NCT00185835 |
| Paclitaxel, Carboplatin, and Gefitinib in Treating Patients With Advanced Non-Small Cell Lung Cancer |
Unknown status |
Lung Cancer |
NCT01024712 |
| Chemotherapy Plus Gefitinib Followed by Chemotherapy, Radiation Therapy, and Gefitinib For Head and Neck Cancer |
Completed |
Head and Neck Cancer |
NCT00193284 |
| Osimertinib Treatment on EGFR T790M Plasma Positive NSCLC Patients (APPLE) |
Recruiting |
NSCLC |
NCT02856893 |
| Safety and Effectiveness Study of Docetaxel and ZD1839 Followed by Removal of the Prostate to Treat Prostate Cancer |
Completed |
Prostate Cancer |
NCT00242918 |
| Safety Trial of IRESSA, Cisplatin and Radiation Therapy for Patients With Head and Neck Cancer |
Terminated |
Head and Neck Cancer |
NCT00195078 |
| Gefitinib (Iressa) in Combo With Chemoradiation in Patients With Locally Advanced Head & Neck Cancer |
Completed |
Head and Neck Neoplasms |
NCT00239304 |
| IRESSA™ In Combo With Xeloda™ in Advanced Colorectal Cancer Patients After 1st-Line Chemo Failure |
Completed |
Colorectal Cancer |
NCT00242788 |
| Iressa vs Placebo as Maintenance Therapy in Locally Advanced NSCLC |
Terminated |
Non Small Cell Lung Carcinoma |
NCT00234468 |
| Immune-Modulated Study of Selected Small Molecules (Gefitinib, AZD9291, or Selumetinib + Docetaxel) or a 1st Immune-Mediated Therapy (IMT; Tremelimumab) With a Sequential Switch to a 2nd IMT (MEDI4736) in Patients With Locally Advanced or Metastatic Non-Small-Cell Lung Cancer |
Completed |
Locally Advanced or Metastatic Non-Small-Cell Lung Cancer (Stage IIIB-IV) |
NCT02179671 |
| First Line Treatment in EGFR Mutation Positive Advanced NSCLC Patients With Central Nervous System Metastases |
Recruiting |
Non-small Cell Lung Cancer|EGFR Gene Mutation|Brain Metastases |
NCT03653546 |
| Chemotherapy for Patients With Non-Small Cell Lung Cancer Who Are Non-Smokers |
Completed |
Non-small Cell Lung Cancer |
NCT00409006 |
| Cisplatin and Irinotecan Chemotherapy, Followed by ZD 1839 (Iressa) in Patients With Esophageal or Gastric Carcinomas |
Completed |
Esophageal Cancer|Gastric Cancer |
NCT00215995 |
| Phase II Neoadjuvant ER+/PgR + Arimidex +/- Iressa Study |
Completed |
Breast Cancer |
NCT00255463 |
| Maintenance ZD1839 (IRESSA®) Following Completion of Front Line, Platinum-Based, Double Chemotherapy in Subjects With Advanced or Metastatic Non-Small Cell Lung Cancer |
Withdrawn |
Non-Small-Cell Lung Carcinoma |
NCT00090675 |
| Gefitinib and Radiation Therapy in Treating Patients With Inoperable Stage I or Stage II Non-Small Cell Lung Cancer |
Withdrawn |
Non-small Cell Lung Cancer |
NCT00268255 |
| A Phase 2 Study of Tomudex & Iressa as Second Line Chemotherapy in Subjects With Colorectal Carcinoma |
Completed |
Colorectal Cancer |
NCT00234429 |
| Dasatinib in Treating Patients With Advanced Lung Cancer That Is No Longer Responding to Erlotinib or Gefitinib |
Completed |
Lung Cancer |
NCT00570401 |
| Study of ZD1839 Combined With Irinotecan and Vincristine in Pediatric Patients With Refractory Solid Tumors |
Completed |
Solid Tumors |
NCT00186979 |
| IRESSA in Combination With Cisplatin & Radiotherapy in Patients With Advanced Head & Neck Carcinoma |
Completed |
Cancer of Head and Neck |
NCT00242749 |
| Capecitabine, Oxaliplatin, and Gefitinib in Treating Patients With Metastatic Colorectal Cancer |
Terminated |
Colorectal Cancer |
NCT00087334 |
| Gemcitabine, Cisplatin, and Gefitinib in Treating Patients Who Are Undergoing Surgery for Stage III Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00103051 |
| ZD1839 (Iressa™) and Concurrent Chemo-Radiation in Patients With Locally Advanced Non Small Cell Lung Cancer |
Completed |
Non Small Cell Lung Carcinoma |
NCT00252798 |
| Increased Dose of Icotinib in Advanced None Small Cell Lung Cancer Patients After Routine Gefitinib Therapy |
Suspended |
Non-small Cell Lung Cancer |
NCT01720901 |
| Iressa in Poor Performance Status Patients With Previously Untreated Advanced Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00193336 |
| Calcitriol and Gefitinib With or Without Dexamethasone in Treating Patients With Advanced Solid Tumors |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00084708 |
| IRESSA Combined With Radiotherapy & Gemcitabine as First-Line Treatment in Locally Advanced Pancreatic Cancer |
Completed |
Pancreatic Cancer |
NCT00234416 |
| Iressa and Radiotherapy in the Treatment of the Locally Advanced Inoperable Squamous Cell Carcinoma of the Head and Neck |
Withdrawn |
Head and Neck Cancer|Carcinoma, Squamous Cell |
NCT00233636 |
| Anastrozole and ZD 1839 in Treating Postmenopausal Women With Metastatic Breast Cancer |
Completed |
Breast Cancer |
NCT00049062 |
| Gilotrif (Afatinib) in Advanced Non-Small Cell Lung Cancer Patients Pre-treated With Erlotinib or Gefitinib |
Completed |
Non-small Cell Lung Cancer |
NCT02747953 |
| A Retrospective Pharmacogenomics Research of EGFR-TKIs,Gefitinib and Erlotinib, in NSCLC Patients Treatment |
Recruiting |
Non-small Cell Lung Cancer (NSCLC)|EGFR-TKI Resistant Mutation|EGFR-TKI Sensitizing Mutation|Germline Mutations|Somatic Mutation |
NCT01994057 |
| Combination Weekly Taxotere™ With Iressa™ /Placebo in Metastatic Breast Cancer |
Completed |
Metastatic Breast Cancer |
NCT00319618 |
| Gefitinib, Cisplatin, Irinotecan, and Radiation Therapy Before Surgery in Treating Patients With Esophageal Cancer or Gastroesophageal Junction Cancer That Can Be Removed By Surgery |
Terminated |
Esophageal Cancer |
NCT00290719 |
| Oxaliplatin, Gefitinib, and Radiation Therapy in Treating Patients With Locally Advanced or Metastatic Esophageal Cancer |
Terminated |
Esophageal Cancer |
NCT00093652 |
| Combination Casodex® and Iressa™ in Locally Advanced Prostate Cancer |
Completed |
Locally Advanced Prostate Cancer |
NCT00319787 |
| An Open Label Study of BIBW 2992/Afatinib in Advanced Non-Small Cell Lung Cancer Patients Pre-treated With Erlotinib or Gefitinib |
Unknown status |
NSCLC |
NCT01932229 |
| Gefitinib and Combination Chemotherapy in Treating Patients With Advanced Solid Tumors or Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00025142 |
| Radiotherapy,Chemotherapy,Before and After Surgery in Advanced Esophageal or Gastroesophageal Junction Cancer |
Completed |
Esophageal Cancer |
NCT00258323 |
| Study of XL647 in Subjects With NSCLC Who Have Progressed After Responding to Treatment With Gefitinib or Erlotinib |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00522145 |
| Tetracycline in Preventing Skin Rash in Patients Who Are Receiving Drugs Such as Gefitinib and Cetuximab for Cancer |
Completed |
Unspecified Adult Solid Tumor, Protocol Specific |
NCT00091247 |
| ZD 1839 Plus Combination Chemotherapy in Treating Patients With Locally Advanced, Locally Recurrent, or Metastatic Colorectal Cancer |
Completed |
Colorectal Cancer |
NCT00026364 |
| BIBW 2992 and BSC Versus Placebo and BSC in Non-small Cell Lung Cancer Patients Failing Erlotinib or Gefitinib (LUX-LUNG 1) |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00656136 |
| A Phase I/II Study of Bexarotene in Combination With ZD1839 (IRESSA®) in the Treatment of Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00238628 |
| ZD 1839 Plus Combination Chemotherapy in Treating Patients With Non-Small Cell Lung Cancer |
Unknown status |
Lung Cancer |
NCT00006048 |
| PharmacoEconomic Assessment IRESSA® in the Treatment of Non-Small-Cell Lung Cancer (NSCLC) |
Unknown status |
Non-Small Cell Lung Cancer |
NCT00173524 |
| A Study on the Long Term Survivals in an Expand Access Program (EAP) of Iressa |
Completed |
Non-small Cell Lung Cancer |
NCT01000740 |
| Pemetrexed Disodium and Carboplatin or Cisplatin With or Without Erlotinib Hydrochloride in Treating Patient With Stage IV Non-Small Cell Lung Cancer Resistant to First-Line Therapy With Erlotinib Hydrochloride or Gefitinib |
Withdrawn |
Recurrent Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer |
NCT01928160 |
| ZD 1839 Plus Chemotherapy in Treating Patients With Non-Small Cell Lung Cancer |
Unknown status |
Lung Cancer |
NCT00006049 |
| AZD9291 Versus Gefitinib or Erlotinib in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer |
Active, not recruiting |
Locally Advanced or Metastatic EGFR Sensitising Mutation Positive Non Small Cell Lung Cancer |
NCT02296125 |
| Phase II Iressa + Irradiation Followed by Chemo in NSCLC |
Completed |
Non-Small Cell Lung Cancer |
NCT00333294 |
| This Study is to Compare the Efficacy of ZD6474 and ZD1839 in Subjects With NSCLC. |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00059722 |
| A Phase II Study of ZD1839 and Tamoxifen in Patients With Epithelial Ovarian Carcinoma, Cancer of the Fallopian Tube or the Peritoneum Refractory to Platinum- and Taxane-based Therapy |
Completed |
Ovarian Cancer|Cancer of the Fallopian Tube|Peritoneal Cancer |
NCT00189358 |
| β-elemene Combine With EGFR-TKI for Advanced EGFR-TKI-resistant NSCLC |
Unknown status |
Non Small Cell Lung Cancer |
NCT03123484 |
| ZD1839 (IRESSA™) in Combination With Docetaxel & Cisplatin in Subjects With Metastatic Head & Neck Cancer |
Completed |
Head and Neck Cancer |
NCT00242762 |
| A Study of BPI-7711 Capsule in Non-small Cell Lung Cancer Patients |
Recruiting |
NSCLC |
NCT03866499 |
| Irinotecan, 5-Fluorouracil and Leucovorin With or Without Iressa in the Treatment of Metastatic Colorectal Cancer |
Withdrawn |
Metastatic Colorectal Cancer |
NCT00233623 |
| Dynamic Changes of Circulating Tumor DNA in Late Stage NSCLC Patients |
Unknown status |
Carcinoma, Non-Small-Cell Lung|Bronchial Neoplasms|Carcinoma, Bronchogenic|Lung Diseases|Lung Neoplasms |
NCT02804100 |
| Iressa Case Control Study in Japan |
Completed |
Non-small Cell Lung Cancer |
NCT00252759 |
| Descriptive Pharmacoepidemiological Study of Patients Treated With Iressa |
Completed |
Lung Cancer |
NCT01448187 |
| LUX-Lung 4: BIBW 2992 (Afatinib) Phase I Trial in Advanced Non Small Cell Lung Cancer Patients & Phase II Trial in Non Small Cell Lung Cancer Patients Failing Erlotinib or Gefitinib |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00711594 |
| Abivertinib Maleate Versus Geifitinib in Patients With Advanced Non-small Cell Lung Cancer With Sensitive EGFR Mutation |
Not yet recruiting |
Advanced Non-small Cell Lung Cancer |
NCT03856697 |
| Study to Allow Patients Previously Participating in a Novartis Sponsored Trial to Continue Receiving INC280 Treatment as Single Agent or in Combination With Other Treatments. |
Active, not recruiting |
Advanced Solid Tumors Which Are cMET-dependent |
NCT03040973 |
| Exploratory Study of Drug Sensitivity Prediction Software (IRCR-DReSS) With Patient-derived Tumor Cells of Metastatic Gastric Cancer |
Recruiting |
Stomach Neoplasms |
NCT03170180 |
| LUX-Lung 5: Afatinib Plus Weekly Paclitaxel Versus Investigator's Choice of Single Agent Chemotherapy Following Afatinib Monotherapy in Non-small Cell Lung Cancer Patients Failing Erlotinib or Gefitinib |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT01085136 |
| Trial of Continuous Once Daily Oral Treatment Using BIBW 2992 (Afatinib) Plus Sirolimus (Rapamune®) in Patients With Non-small Cell Lung Cancer Harbouring an EGFR Mutation and/or Disease Progression Following Prior Erlotinib or Gefitinib |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00993499 |
| Stereotactic Body Radiation Therapy (SBRT) in Newly Diagnosed Advanced Staged Lung Adenocarcinoma (Sindas) |
Recruiting |
Stage IV EGFR Mutated Non-Small Cell Lung Cancer |
NCT02893332 |
| Anticancer Activity of Nicotinamide on Lung Cancer |
Active, not recruiting |
Non-Small-Cell Lung Carcinoma |
NCT02416739 |
| EGFR-TKIs Combine With Anlotinib as First-line Treatment for Patients With Advanced EGFR Mutation-positive NSCLC |
Recruiting |
EGFR Gene Mutation |
NCT03720873 |
| EGFR-TKI With/Without Chemotherapy in NSCLC Patients With Both EGFR Mutation and BIM Deletion Polymorphism |
Unknown status |
Nonsmall Cell Lung Cancer|EGFR Gene Mutation |
NCT03002844 |
| A Study of LY2875358 in Japanese Participants With Advanced Cancer |
Completed |
Solid Tumors|Lymphoma|Carcinoma, Non-Small-Cell Lung |
NCT01602289 |
| Customized Neoadjuvant Versus Standard Chemotherapy in NSCL Patients With Resectable Stage IIIA (N2)Disease |
Unknown status |
Non Small Cell Lung Cancer |
NCT01784549 |
| Apatinib Combine With EGFR-TKI for Advanced EGFR-TKI-resistant Non-Small Cell Lung Cancer |
Unknown status |
Nonsmall Cell Lung Cancer |
NCT03050411 |
| Gemcitabine/Irinotecan/ZD1839 vs Paclitaxel/Carboplatin/Etoposide/ZD1839 in Carcinoma of Unknown Primary Site |
Completed |
Neoplasms, Unknown Primary |
NCT00193596 |
| A Study of Hypofractionated Radiotherapy for Limited Metastatic NSCLC Harboring Sensitizing EGFR Mutations After First Line TKI Therapy |
Not yet recruiting |
Lung Adenocarcinoma|EGFR Positive Non-small Cell Lung Cancer |
NCT02788058 |
| A Open-label Prospective Cohort Trial of Curcumin Plus Tyrosine Kinase Inhibitors (TKI) for EGFR -Mutant Advanced NSCLC |
Unknown status |
Lung Cancer |
NCT02321293 |
| Study of Chemotherapy Sequenced by or Combined With EGFR-TKIs for NSCLC Patients Failed to EGFR-TKIs Therapy |
Unknown status |
Non Small Cell Lung Cancer |
NCT01746277 |
| Can Epidermal Growth Factor Receptor Improve the Postoperative Survivorship for Inoperable Non-small Cell Lung Cancer With Spinal Metastasis ? |
Completed |
Is Targeted Therapy Increasing Survival Inoperable Nonsmall Cell Lung Cancer With Spinal Metastasis ? |
NCT02740894 |
| Induction Chemotherapy Using Paclitaxel, Carboplatin, CPT-11 With Pegfilgrastim |
Completed |
Non Small Cell Lung Cancer |
NCT00280787 |
| Anlotinib Hydrochloride Combined With EGFR TKIs in Advanced Non-small Cell Lung Cancer |
Not yet recruiting |
Non-Small-Cell Lung |
NCT03766490 |
| Trial of BIBW 2992 (Afatinib) + Cetuximab in Non-Small Cell Lung Cancer |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT01090011 |
| A Study of Ramucirumab (LY3009806) in Combination With Erlotinib in Previously Untreated Participants With EGFR Mutation-Positive Metastatic NSCLC (RELAY) |
Active, not recruiting |
Metastatic Non-Small Cell Lung Cancer |
NCT02411448 |
| Intercalated Combination of Chemotherapy and Tyrosine Kinase Inhibitors as First-line Treatment for Patients With Non-Small-Cell Lung Cancer |
Unknown status |
Non-Small-Cell Lung Cancer |
NCT02031601 |
| Afatinib Plus Nimotuzumb for NSCLC |
Unknown status |
NSCLC |
NCT01861223 |
| EGFR-TKI Concurrent With/Without WBRT in Brain Metastasis From NSCLC |
Recruiting |
Non-Small Cell Lung Cancer |
NCT02714010 |
| Radiation Therapy to the Brain or Observation in Preventing Brain Metastases in Patients With Advanced Non-Small Cell Lung Cancer |
Unknown status |
Lung Cancer |
NCT00955695 |
| Efficacy Study of Chinese Medicine Plus EGFR-TKI Versus EGFR-TKI in Advanced Pulmonary Adenocarcinoma |
Unknown status |
Cancer |
NCT01745302 |
| Therapy for Children With Neuroblastoma |
Completed |
Neuroblastoma |
NCT00135135 |
| Clinical Study of Chinese Medicine Plus Targeted Therapy Maintenance in Advanced Pulmonary Adenocarcinoma |
Completed |
Cancer |
NCT02889692 |
| Combination Chemotherapy in Treating Patients With Advanced Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00005806 |
| Anlotinib Hydrochloride Combined With EGFR-Tyrosine Kinase Inhibitor (TKI) in Treating Advanced NSCLC Patients With Acquired Resistance to EGFR-TKI |
Not yet recruiting |
NSCLC |
NCT04007835 |
| Hypofractionated Brain Radiation In EGFR Mutated Adenocarcinoma Cranial Disease (Hybrid) |
Recruiting |
Stage IV EGFR Mutated NSCL With Brain Metastases |
NCT02882984 |
| Evaluation of EGFR TKI Resistance Mechanism Using Plasma DNA Analysis |
Unknown status |
EGFR Mutation Positive Non-small Cell Lung Cancer |
NCT01776684 |
| Radiotherapy Combined With Thymosin for Metastatic NSCLC Patients Who Showed Stable Disease After First Line TKI Therapy |
Recruiting |
Lung Adenocarcinoma |
NCT02787447 |
| Apatinib in the Treatment of Patients With EGFR T790M-Negative NSCLC |
Not yet recruiting |
Lung Diseases|Neoplasms|Respiratory Tract Diseases|Thoracic Neoplasms|Non-Small-Cell Lung |
NCT03389256 |
| Study of EGF816 in Combination With Selected Targeted Agents in EGFR-mutant NSCLC |
Recruiting |
EGFR-mutant Non-small Cell Lung Cancer |
NCT03333343 |
| A Study Of Combined C- MET Inhibitor And PAN-HER Inhibitor (PF-02341066 And PF-00299804) In Patients With Non- Small Cell Lung Cancer |
Completed |
Non Small Cell Lung Cancer |
NCT01121575 |
| Lung Cancer in Women Treated With Anti-oestrogens anD Inhibitors of EGFR |
Active, not recruiting |
Stage IV Lung Cancer |
NCT01556191 |
| Randomized Phase 2 Study of 3 Therapeutic Modalities in PS 2/3 Patients With NSCLC Stage IIIB/IV |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT00198393 |
| Retreatment With Epidermal Growth Factor Receptor(EGFR) Tyrosine Kinase Inhibitor in EGFR Mutation Positive Patients |
Recruiting |
EGFR Positive Non-small Cell Lung Cancer |
NCT03382795 |
| Local Non-salvage Radiotherapy for Synchronous Oligometastatic Non-small-cell Lung Cancer. |
Recruiting |
Stage IV Non-small Cell Lung Cancer |
NCT03119519 |
| Phase 2 Platform Study in Patients With Advanced Non-Small Lung Cancer Who Progressed on First-Line Osimertinib Therapy (ORCHARD) |
Recruiting |
Non-Small Cell Lung Cancer |
NCT03944772 |
| Anti-angiogenesis Combine With EGFR-TKI in Advanced Non-squamous Non Small Cell Lung Cancer |
Unknown status |
Non Small Cell Lung Cancer |
NCT03461185 |
| EGFR-TKI With Chemotherapy in NSCLC Patients With Both EGFR Mutation and BIM Deletion Polymorphism |
Unknown status |
Non-Small-Cell Lung Cancer |
NCT02859077 |
| De Novo Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors |
Unknown status |
NSCLC |
NCT01697163 |
| Studying Biomarkers in Samples From Patients With Recurrent or Metastatic Head and Neck Cancer Treated on E1302 Trial |
Completed |
Head and Neck Cancer |
NCT01619618 |
| Study of Endostatin Combined With Docetaxel in the Treatment of Advanced Non-small Cell Lung Cancer Patients |
Unknown status |
Carcinoma, Non-Small-Cell Lung |
NCT01192230 |
| Maintenance Targeted Therapy With or Without Stereotactic Body Radiation Therapy for Stage IV Non-small Cell Lung Cancer |
Recruiting |
Stage IV Non-small Cell Lung Cancer |
NCT03595644 |
| Concordance of Key Actionable Genomic Alterations as Assessed in Tumor Tissue and Plasma in Non Small Cell Lung Cancer |
Terminated |
Non Small Cell Lung Carcinoma |
NCT02762877 |
| A Pilot Study of Genomic Sequencing Guided Individualized Therapy in Gastrointestinal Cancers, GITIC Study |
Unknown status |
Gastrointestinal Cancers |
NCT02013089 |
| Trial Of PF-00299804 In Patients With Advanced Refractory Lung Cancer |
Completed |
Carcinoma, Non Small Cell Lung |
NCT00553254 |
| Psychometric Properties of the EORTC QLQ-LC29 |
Completed |
Lung Cancer |
NCT02745691 |
| A Study of Pembrolizumab (MK-3475) in Combination With Chemotherapy or Immunotherapy in Participants With Non-small Cell Lung Cancer (MK-3475-021/KEYNOTE-021) |
Active, not recruiting |
Non-small Cell Lung Carcinoma |
NCT02039674 |
| Study Evaluating the Safety Of HKI-272 (Neratinib) In Subjects With Advanced Non-Small Cell Lung Cancer |
Completed |
Carcinoma, Non-Small-Cell Lung|Lung Neoplasms |
NCT00266877 |
| ARQ 197 Plus Erlotinib in Patient With Locally Advanced or Metastatic EGFR Mutation-positive Non-small-cell Lung Cancer |
Completed |
Non-small-cell Lung Cancer |
NCT01580735 |
| Efficacy and Safety of Targeted Precision Therapy in Refractory Tumor With Druggable Molecular Event |
Recruiting |
Rare Tumor|Refractory Tumor |
NCT03239015 |
| Development of Circulating Tumour Cell Molecular Diagnostics Using a Novel Microfluidic Device |
Unknown status |
Patients With Non-small Cell Lung Cancer |
NCT01193829 |
| First Line Bio-immunotherapy With Thymosin Alpha 1 in Patients With Sensitizing EGFR Mutation Positive Non Small Cell Lung Cancer Who Are Taking Standard of Care Therapy |
Unknown status |
EGFR Mutation Positive Non Small Cell Lung Cancer |
NCT02906163 |
| Evaluation of the High Dose of Icotinib in Advanced Lung Cancer Patients After Failure of Target Therapy |
Unknown status |
NSCLC |
NCT01963195 |
| Conmana Combined With Thalidomide to Treat NSCLC |
Unknown status |
NSCLC |
NCT02778893 |
| Tyrosine Kinase Inhibitors In Metastatic Non Small Cell Lung Cancer |
Unknown status |
Non Small Cell Lung Cancer |
NCT01163058 |
| Study to Assess the Effect of Itraconazole (a CYP3A4 Inhibitor) on the Pharmacokinetics of AZD9291, in Patients With EGFR Positive Non-small Cell Lung Cancer. Patients Will be Chosen From Those Who Have Already Been Prescribed an EGFR TKI Medicine (Such as Iressa or Tarceva) |
Active, not recruiting |
Advanced Non Small Cell Lung Cancer|Advanced (Inoperable) Non Small Cell Lung Cancer |
NCT02157883 |
| Neoadjuvant Therapy in Resectable Non-Small Cell Lung Cancer Stages IIIA |
Recruiting |
Non Small Cell Lung Cancer |
NCT04197076 |
| Evaluation of the Efficacy of Low-dose Acetylsalicylic Acid on Diarrhea Induced by Anti-cancer Targeted Therapies. |
Withdrawn |
Cancer |
NCT02323516 |
| The Evaluation of Lung Cancer Patient Treated With Epidermal Growth Factor Receptor Tyrosine Kinase |
Unknown status |
Non-Small-Cell Lung Cancer |
NCT02755337 |
| A Phase II Trial of Afatinib in Patients With Metastatic or Recurrent Squamous Cell Carcinoma of Esophagus |
Active, not recruiting |
Squamous Cell Carcinoma of Esophagus |
NCT02353936 |
| Tetracycline as a Prophylaxis for Rash in Patients With NSCLC Receiving Treatment With BIBW2992 (Afatinib) |
Completed |
Skin Rash|Lung Cancer |
NCT01880515 |
| Efficacy and Safety Study of Bevacizumab Plus Chemotherapy in EGFR-TKI Resistant Non-Squamous Non-Small Cell Lung Cancer |
Unknown status |
Lung, Carcinoma |
NCT02139579 |
| Clinical Pharmacokinetics of TKIs in Chinese Patients of Hepatitis B (HBV) |
Recruiting |
Non Small Cell Lung Cancer|Hepatitis B |
NCT03680183 |
| Circulating Tumor DNA (ctDNA) as a Prognostic Tool in Patients With Advanced Lung Adenocarcinoma |
Unknown status |
Adenocarcinoma of Lung (Disorder) |
NCT03090815 |
| Extension Study of Lapatinib Plus Herceptin With or Without Endocrine Therapy |
Active, not recruiting |
Breast Cancer |
NCT00999804 |
| Erlotinib as 1st Line Treatment in NSCLC Stage IIIB/IV |
Completed |
Non Small Cell Lung Cancer |
NCT00615758 |
| LUX Lung Special Access Scheme Australia Named Patient Use (NPU) |
Approved for marketing |
Carcinoma, Non-Small-Cell Lung |
NCT01209650 |
| Afatinib Plus Chemotherapy Against Esophageal or Lung Squamous Cell Carcinoma |
Not yet recruiting |
Squamous Cell Carcinoma |
NCT03486509 |
| Intermittent and Maintenance of Icotinib in Combination With Pemetrexed/Carboplatin Compared With Icotinib Single Drug in â…˘b/IV Non Small Cell Lung Cancer With Epidermal Growth Factor Receptor (EGFR) Mutation |
Not yet recruiting |
Lung, Carcinoma |
NCT03151161 |
| Intermittent and Maintenance of Erlotinib in Combination With Pemetrexed/Carboplatin in â…˘b/IV Non Small Cell Lung Cancer With Epidermal Growth Factor Receptor (EGFR) Mutation |
Unknown status |
Lung, Carcinoma |
NCT02066038 |
| Osimertinib With or Without Bevacizumab for EGFR- Mutant Non-small Cell Lung Cancer With Leptomeningeal Metastasis |
Not yet recruiting |
Leptomeningeal Metastasis|Non-small Cell Lung Cancer|EGFR Activating Mutation |
NCT04148898 |
| Registry for Patients With Acquired Resistance to Small Molecule Kinase Inhibitors in Non-Small-Cell Lung Cancer |
Completed |
Unresectable or Metastatic Non-small Cell Lung Cancer (NSCLC) |
NCT00579683 |
| Phase II Clinical Study of Intermittent High Dose of Icotinib in Combination With Docetaxel to Treat Lung Cancer |
Unknown status |
Non Small Cell Lung Cancer |
NCT02191059 |
| Pemetrexed and Erlotinib for Metastatic Colorectal Cancer |
Completed |
Metastatic Colorectal Cancer |
NCT02723578 |
| Uncommon EGFR AZD9291 |
Unknown status |
Non Small Cell Lung Cancer |
NCT03424759 |
| Combination of Cetuximab With Afatinib for Patient With EGFR Mutated Lung Cancer |
Active, not recruiting |
Non Small Cell Lung Cancer |
NCT02716311 |
| PF-00299804 in Patients With Head and Neck Squamous Cell Carcinoma |
Completed |
Head Neck Cancer Squamous Cell Metastatic|Head Neck Cancer Squamous Cell Recurrent |
NCT01449201 |
| MEchanisms of Resistance in EGFR Mutated Nonpretreated Advanced Lung Cancer Receiving OSimErtib |
Recruiting |
Non-small Cell Lung Cancer |
NCT03865511 |
| Study Investigating the Effect of Everolimus Monotherapy in Patients With Advanced Non-small Cell Lung Cancer (NSCLC) |
Completed |
Non-small-cell Lung Carcinoma |
NCT00124280 |
| Cetuximab in Patients With Lung Adenocarcinoma Receiving Erlotinib That Have Developed "Acquired Resistance" to Erlotinib |
Completed |
Lung Adenocarcinoma |
NCT00716456 |
| Local Ablative Therapy for Treatment of Oligoprogressive, EGFR-Mutated, Non-Small Cell Lung Cancer After Treatment With Osimertinib |
Active, not recruiting |
Lung Adenocarcinoma|Lung Neoplasms |
NCT02759835 |
| A Phase II Study of CI-1033 in Treating Patients With Metastatic (Stage IV) Breast Cancer |
Completed |
Breast Neoplasms |
NCT00051051 |
| Endostar Combination With Chemotherapy and EGFR-TKI in Lung Cancer Rechallenging Treatment After Acquired Resistance. |
Unknown status |
Lung Cancer |
NCT02350361 |
| A Window of Opportunity Trial of Afatinib In Early Stage Non-Small Cell Lung Cancer (NSCLC) |
Withdrawn |
Lung Cancer |
NCT01480141 |
| A Study of LDK378 in Patients With Non-small Cell Lung Cancer Harboring ROS1 Rearrangement |
Recruiting |
Non-small Cell Lung Cancer Harboring ROS1 Rearrangement |
NCT03399487 |
| Survival Outcomes and Tumor Molecular Profile Following Bicalutamide Neoadjuvant Therapy |
Completed |
Prostate Cancer |
NCT00418080 |
| Phase 2 Study of AUY922 in NSCLC Patients With Exon 20 Insertion Mutations in EGFR |
Completed |
Non Small Cell Lung Cancer |
NCT01854034 |
| A Phase II Trial of PF-00299804 in Patients With Metastatic or Recurrent Squamous Cell Carcinoma of Esophagus |
Completed |
Esophageal Squamous Cell Carcinoma |
NCT01608022 |
| Clinical Trial to Evaluate Pharmacokinetics,Safety and Immunogenicity of Single Injection of CDP1 to Healthy Volunteers Compared to Erbitux |
Recruiting |
Healthy Volunteers |
NCT03881787 |
| A Positron Emission Tomography (PET) Imaging Agent [18F]-ODS2004436 as a Marker of EGFR Mutation in Subjects With NSCLC |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT02847377 |
| A Preliminary Clinical Study on the Pharmacokinetics and Safety of CDP1 in Patients With Advanced CRC or HNSCC |
Active, not recruiting |
Colorectal Cancer |
NCT03491709 |
| Dasatinib and Osimertinib (AZD9291) in Advanced Non-Small Cell Lung Cancer With EGFR Mutations |
Active, not recruiting |
EGFR Gene Mutation|Nonsmall Cell Lung Cancer |
NCT02954523 |
| Liver Function Test (LFT) Elevations in Cancer Patients and Users of Tyrosine Kinase Inhibitor (TKI) Drugs |
Completed |
Cancer |
NCT01098500 |
| Phase II Trial of HM781-36B in Patients With Metastatic/Recurrent Head and Neck Squamous Cell Carcinoma (HNSCC) After Failure of or Unfit for Platinum-containing Therapy |
Recruiting |
HNSCC |
NCT02216916 |
| Phase II Study of Afatinib as Third- or Further-line Treatment for Patients With Stage IV Bronchial Adenocarcinoma, Harboring Wild-type EGFR, Expressing the Neurotensin - Neurotensin Receptor Complex |
Withdrawn |
SMALL CELL LUNG CARCINOMA |
NCT02876081 |
| A Retrospective Study of Biomarkers in Non-Small Cell Lung Cancer |
Unknown status |
Non Small Cell Lung Cancer |
NCT01100840 |
| Metformin Plus TKI Use in Patients With Non-Small Cell Lung Carcinoma |
Unknown status |
Non-Small Cell Adenocarcinoma|Tyrosine Kinase Mutation|EGFR Gene Mutation |
NCT03071705 |
| TAURAS - T790 AURA ScreenFailure SOC Registry Study |
Terminated |
Non Small Cell Lung Cancer |
NCT02405247 |
| Low Dose Daily Erlotinib in Combination With High Dose Twice Weekly Erlotinib in Patients With EGFR-Mutant Lung Cancer |
Completed |
EGFR-Mutant Lung Cancer |
NCT01967095 |
| Icotinib Combined With Whole Brain Radiotherapy in Treating Multiple Brain Metastases From Non-Small Cell Lung Cancer |
Completed |
Lung Cancer|Metastatic Cancer |
NCT01514877 |
| Metformin in Stage IV Lung Adenocarcinoma |
Terminated |
Non-small Cell Lung Cancer |
NCT01997775 |
| The Role of Brain Radiotherapy in Patients With Asymptomatic Brain Metastasis in the Era of Targeted Therapy for NSCLC |
Not yet recruiting |
Non Small Cell Lung Cancer|Brain Metastases |
NCT04193007 |
| IMRT and Timing in Combination With EGFRTKI for Stage IV Non-small-cell Lung Cancer |
Not yet recruiting |
Non-Small Cell Lung Cancer|Nonsmall Cell Lung Cancer|Carcinoma, Non-Small-Cell Lung |
NCT03258671 |
| Molecularly Target Therapy With FORFIRINOX in Advanced or Recurrent Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma |
Recruiting |
Cholangiocarcinoma of the Extrahepatic Bile Duct|Gallbladder Cancer |
NCT03768375 |
| Retrospective Study in Non Small Cell Lung Cancer (NSCLC) With Epidermal Growth Factor Receptor (EGFR) |
Completed |
NSCLC Non Small Cells Lung Cancer |
NCT02293733 |
| Observational Study of Patients With Locally Advanced or Metastatic NSCLC (Non-Small Cell Lung Cancer) |
Terminated |
Carcinoma, Non-Small-Cell Lung |
NCT03053297 |
| Intracranial Activity of AZD9291 (TAGRISSO) in Advanced EGFRm NSCLC Patients With Asymptomatic Brain Metastases |
Recruiting |
Lung Cancer |
NCT02736513 |
| Genomic Based Assignment of Therapy in Advanced Urothelial Carcinoma |
Completed |
Urothelial Carcinoma|Bladder Cancer|Urinary Bladder Neoplasms |
NCT02788201 |
| Epidermal Growth Factor Receptor and K-ras Mutations in Patients With Stage III Non-Small Cell Lung Cancer |
Completed |
Lung Cancer |
NCT00898924 |
| N-of-1 Trial: Actionable Target Identification in Metastatic Cancer for Palliative Systemic Therapy |
Completed |
Metastatic Cancer |
NCT02142036 |
| Treatment Patterns, Outcomes and Testing in EGFRm NSCLC Patients With EGFR TKI 1L Across Europe (REFLECT) |
Completed |
Lung Cancer |
NCT04031898 |
| Vitamin C and Tyrosine Kinase Inhibitor in Lung Cancer Patients With Epidermal Growth Factor Receptor Mutations |
Recruiting |
Carcinoma, Non-Small-Cell Lung |
NCT03799094 |
| Molecularly Target Therapy With GEMOX in Advanced or Recurrent Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma |
Recruiting |
Cholangiocarcinoma of the Extrahepatic Bile Duct|Gallbladder Cancer |
NCT02836847 |
| Molecular Phenotype Changes and Personalized Treatment for CRPC |
Unknown status |
Hormone Refractory Prostate Cancer |
NCT02208583 |
| Survey on the Treatment Reality of Patients With Epidermal Growth Factor Receptor (EGFR) Mutation-positive Non-small Cell Lung Cancer(NSCLC) |
Completed |
Carcinoma, Non-Small-Cell Lung |
NCT02475720 |
| Low-frequency Rotating Magnetic System Combined With Systemic Anti-tumor Therapy for Advanced Lung Cancer |
Recruiting |
Lung Neoplasms |
NCT02701231 |
| Radical Treatment of Synchronous Oligometastatic Non-Small Cell Lung Carcinoma |
Unknown status |
Carcinoma, Non-Small-Cell Lung|Synchronous Neoplasms |
NCT02805530 |
| Evaluating Preventive Therapy With Oint Threolone, Synthomycine or Aqua Cream, for EGFR'I Induced Acneiform Rash |
Completed |
Acneiform Rash |
NCT01256437 |
| A Study of DS-2248, in Subjects With Advanced Solid Tumors |
Terminated |
Solid Tumors|Non-small Cell Lung Carcinoma |
NCT01288430 |
| PD-1 Immune Checkpoint Inhibitors and Immune-Related Adverse Events: a Cohort Study |
Not yet recruiting |
Carcinoma, Non-Small-Cell Lung|Immune-Related Adverse Events |
NCT04115410 |
| High Throughput Drug Sensitivity Assay and Genomics- Guided Treatment of Patients With Relapsed or Refractory Acute Leukemia |
Recruiting |
Acute Leukemia of Ambiguous Lineage|Recurrent Adult Acute Lymphoblastic Leukemia|Recurrent Adult Acute Myeloid Leukemia|Recurrent Childhood Acute Lymphoblastic Leukemia|Recurrent Childhood Acute Myeloid Leukemia|Refractory Acute Myeloid Leukemia|Refractory Adult Acute Lymphoblastic Leukemia|Refractory Childhood Acute Lymphoblastic Leukemia |
NCT02551718 |
| Molecular Profiling and Matched Targeted Therapy for Patients With Metastatic Melanoma |
Not yet recruiting |
Melanoma |
NCT02645149 |